Session Information
Session Type: ACR Late-breaking Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Therapeutic options for diffuse cutaneous systemic sclerosis (dcSSc) are limited. The Scleroderma: Cyclophosphamide or Transplantation (SCOT) trial was a multicenter study designed to evaluate the long-term benefit of myeloablative autologous HSCT compared to 12 monthly pulses of CYC.
Methods: Adults (18-69 years) with dcSSc (ACR criteria) and high-risk lung and/or renal involvement were randomized to CYC (750 mg/m2/mo.) or myeloablation (800 cGy total body irradiation with lung and kidney shielding, 120 mg/kg CYC and 90 mg/kg antithymocyte globulin) followed by CD34+selected autologous HSCT. The primary endpoint was a global rank composite score (GRCS) at 54 months that ordered subjects based on a hierarchy of outcomes: death, event-free survival (EFS), forced vital capacity (FVC), scleroderma health assessment questionnaire, and modified Rodnan skin score (mRSS).
Results: Seventy-five subjects were randomized (39 CYC, 36 HSCT) with mean baseline mRSS=30, mean FVC=74% and mean DLC0=53% of predicted. All but two had lung involvement. In the ITT (randomized) sample, GRCS comparisons favored HSCT and met the predefined primary endpoint at 54 months (p=0.013) and 48 months (p=0.008). In those who were transplanted or received ≥ 9 CYC doses (34 CYC, 33 HSCT), GRCS results (figure 1) were even stronger (p=0.004 and 0.003, respectively). Secondary analyses in treated subjects supported the primary results: at 54 months, EFS was 50% in CYC and 79% in HSCT (p=0.021) and overall survival (OS) was 77% and 91% (p=0.19), respectively. Treatment related mortality at 54 months was 0% CYC and 3% HSCT. Among HSCT recipients, 9% had initiated DMARDs by 54 months compared to 44% of CYC (p=0.001). As shown in figure 2, Kaplan-Meier estimates for EFS and OS differed significantly between groups (p=0.030, 0.019, respectively). The rate of SAEs was similar in both arms. Grade 3 and above AEs including regimen-related cytopenias were more common in the HSCT arm. Herpes zoster was more frequent in the HSCT arm.
Conclusion: This study demonstrates long-term superiority of myeloablative autologous HSCT over CYC on efficacy endpoints, and shows a significant reduction in use of DMARDS among HSCT recipients. Transplant-related mortality was lower and overall survival was better than projected both during the peri-transplant period and long term. The SCOT trial supports myeloablative HSCT as a significant advance in the care of dcSSc. Funded by NIAID; ClinicalTrials.gov NCT00114530 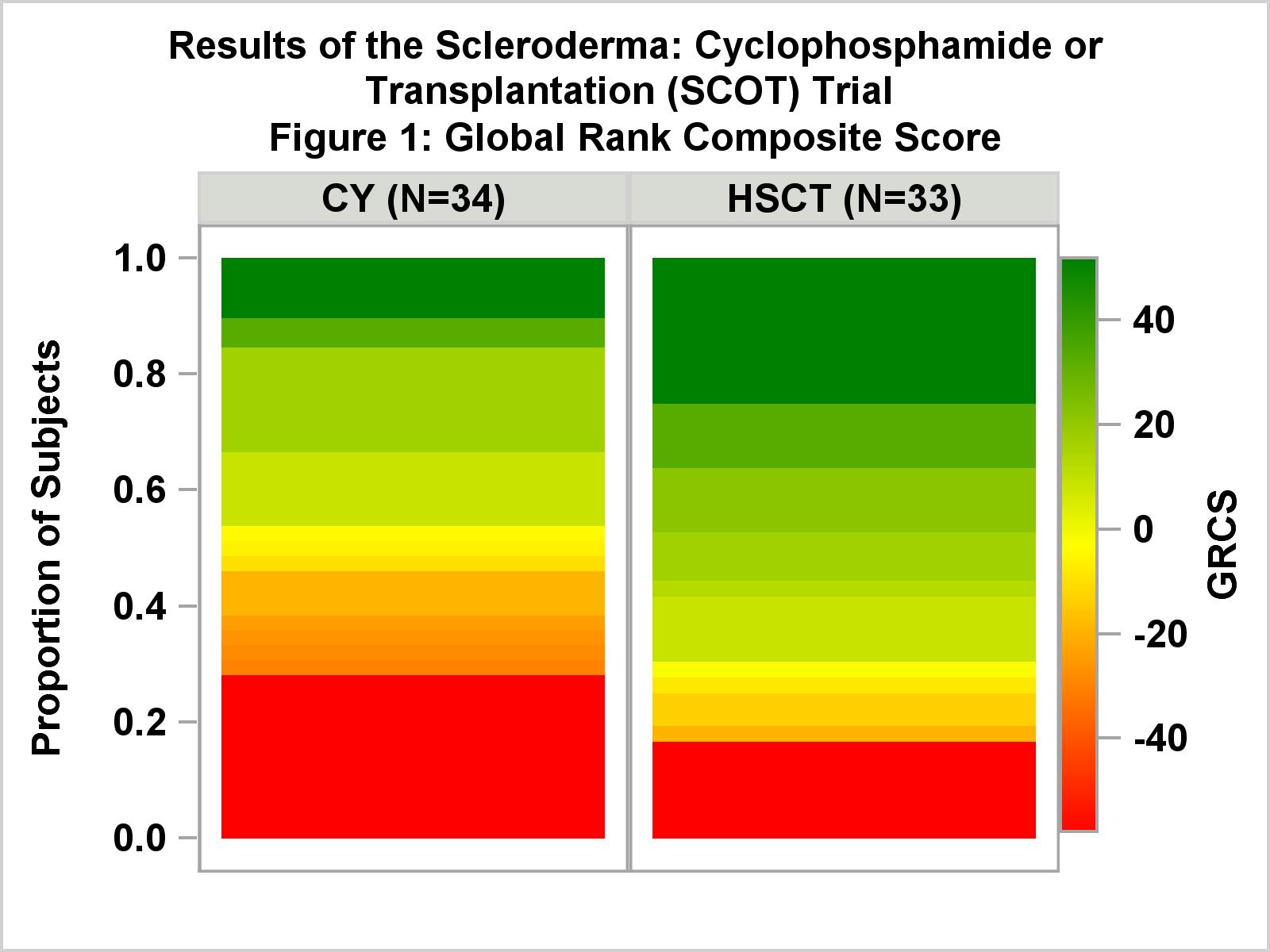

To cite this abstract in AMA style:
Sullivan K, Keyes-Elstein L, McSweeney P, Pinckney A, Welch B, Mayes MD, Nash R, Crofford LJ, Castina S, Griffith L, Goldmuntz E, Furst DE. Myeloablative Autologous Transplantation of CD34+ -Selected Hematopoietic Stem Cells (HSCT) Vs Monthly Intravenous Cyclophosphamide (CYC) for Severe Scleroderma with Internal Organ Involvement: Outcomes of a Randomized North American Clinical Trial [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/myeloablative-autologous-transplantation-of-cd34-selected-hematopoietic-stem-cells-hsct-vs-monthly-intravenous-cyclophosphamide-cyc-for-severe-scleroderma-with-internal-organ-involvement-outcom/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/myeloablative-autologous-transplantation-of-cd34-selected-hematopoietic-stem-cells-hsct-vs-monthly-intravenous-cyclophosphamide-cyc-for-severe-scleroderma-with-internal-organ-involvement-outcom/
