Session Information
Date: Tuesday, November 12, 2019
Title: Miscellanous Rheumatic & Inflammatory Disease Poster III: Autoimmune Conditions and Therapies
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Immune checkpoint inhibitors (ICI) are new anti-cancer agents used for lung cancer, melanoma, renal cell and urothelial carcinoma, and head and neck cancers. They activate anti-tumor immunity which hampers self-tolerance leading to many immune related adverse events (irAE) commonly affecting the skin, endocrine organs and GI tract. Historically, rheumatologists report limited experience and confidence in the management of irAEs. We report our single-center experience with musculoskeletal irAEs.
Methods: University of Pittsburgh melanoma patients treated with ≥1 dose of a PD-1 inhibitor between 2011-2018 were analyzed. Demographic data, cancer diagnosis and treatment and the type and treatment of irAEs were obtained via retrospective chart review.
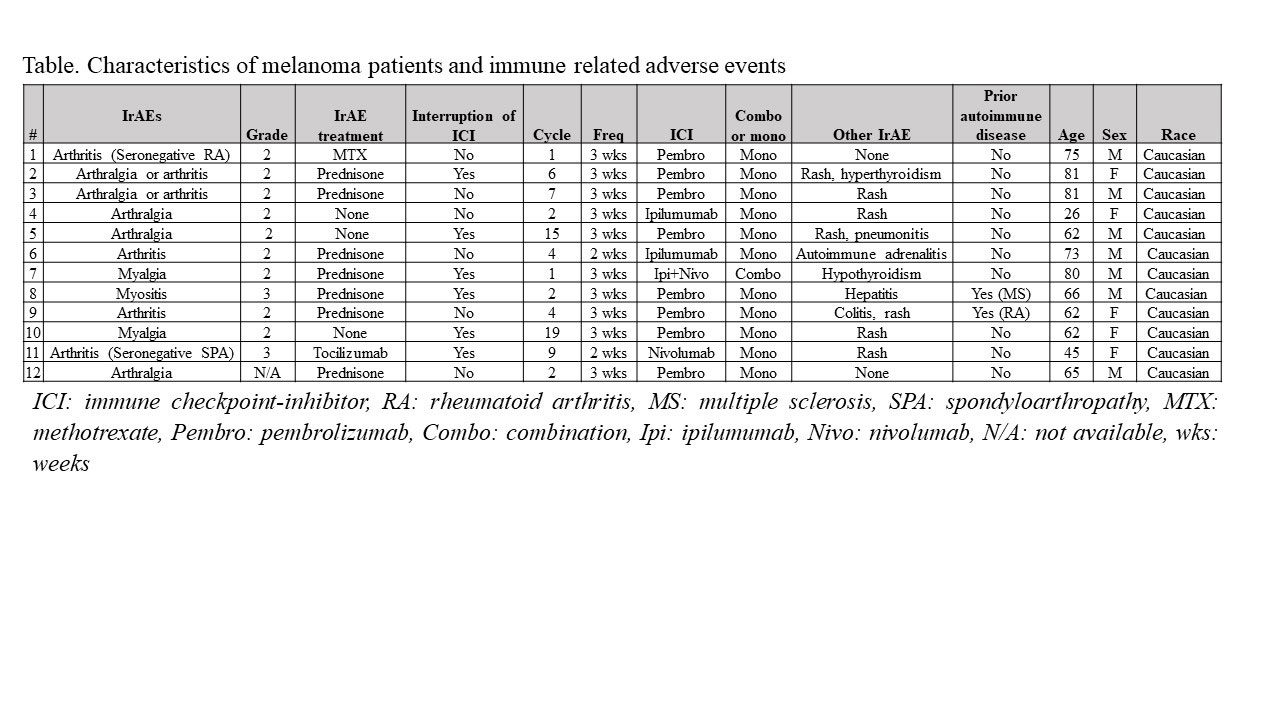
Results: The melanoma cohort included 130 patients with 12 (9.2%) developing ≥ grade 2 arthralgia, arthritis, myalgia or myositis. The mean age was 64.8 (± 16.1) and included 7 males and 5 females. Nine subjects developed ≥ grade 2 arthralgia/arthritis (6.9%) and two had grade 2 myalgias (1.5%) while there was 1 case of myositis (0.7%). Fifty percent of patients had their cancer treatment interrupted and the patient developing myositis was hospitalized. IrAEs occurred after a median of 4 (IQR 2-7.5) cycles and 66 (IQR 20.2-145) days after initiation of cancer treatment and was grade 2 in 81% of cases. Cancer treatment included pembrolizumab (n:8), ipilimumab (n:3) and nivolumab (n:1) in subjects with irAEs, and was single agent in 92% of the cases. Eighty-three percent of the cases developed other non-musculoskeletal irAEs, with rash (80%) the most common followed by thyroid abnormalities (20%). One melanoma patient with pre-existing rheumatoid arthritis (RA) flared with synovitis after 19 ICI cycles and was treated with a short course of prednisone. Treatment of the irAEs included prednisone alone in 58% and at least one additional agent in in 2 (17%) of the cases, all of whom significantly improved. Of the latter 2 cases, 1 was diagnosed with seronegative RA that responded to methotrexate while the other patient had a new diagnosis of seronegative spondyloarthropathy treated with sulfasalazine (discontinued due to rash), methotrexate (ineffective) and later responded to tocilizumab. The subject with myositis was hospitalized with muscle weakness and an elevated creatine kinase (CK) of 1058 U/L and responded to glucocorticoids. Two patients with myalgias and a normal CK improved with ICI discontinuation in one and a short course of prednisone with ICI monotherapy in the second case.
Conclusion: In summary, approximately 10% of melanoma patients receiving immunotherapy developed musculoskeletal irAEs with 50% requiring interruption in cancer treatment, and most (75%) requiring glucocorticoids or other immunosuppressive therapy. Musculoskeletal irAEs responded well to prednisone alone in 80% of cases, while 20% required an additional immunosuppressive agent. As more ICIs are used, it will be necessary to conduct prospective assessments of toxicity in order to better ascertain the frequency as well as to optimally manage the irAES to allow for continuation of effective cancer therapy.
To cite this abstract in AMA style:
Saygin D, Bastacky M, Mascara G, Brenner T, Oddis C, Moghadam-Kia S, Ascherman D, Davar D, Aggarwal R. Musculoskeletal Immune-Related Adverse Events After Immune Checkpoint Inhibitor Therapy: Single Center Experience [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/musculoskeletal-immune-related-adverse-events-after-immune-checkpoint-inhibitor-therapy-single-center-experience/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/musculoskeletal-immune-related-adverse-events-after-immune-checkpoint-inhibitor-therapy-single-center-experience/