Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Enerceptan® (EtaBS) has been developed as a proposed biosimilar of etanercept. Phase I study demonstrated pharmacokinetic equivalence with EtaRef.
Methods: A multicenter, non-inferiority, randomized, assessor -blinded, parallel-group, controlled study was conducted in Argentina (NCT03332719). Adults with active moderate or severe RA (2010 ACR/EULAR criteria) with inadequate response to MTX (stable for ≥90 days and throughout the study) were included. Active RA was defined as ≥6 painful joints and ≥8 swollen joints (using 68 and 66-joint assessment respectively) and DAS28 (ESR) ≥ 3.2 and at least one erosion in hands or feet on X-ray at entry. Subjects were randomized in a 2:1 ratio to 32 weeks treatment with EtaBS or EtaREF in a weekly 50 mg dose subcutaneously. Stratification was based on prior use of biological DMARDS and concomitant use of steroids. The primary efficacy endpoint was ACR20 response rate at week 32. Safety, immunogenicity and steady state concentration of both drugs was evaluated. The non-inferiority margin for ACR20 was estimated in 12% with a power of 80%.
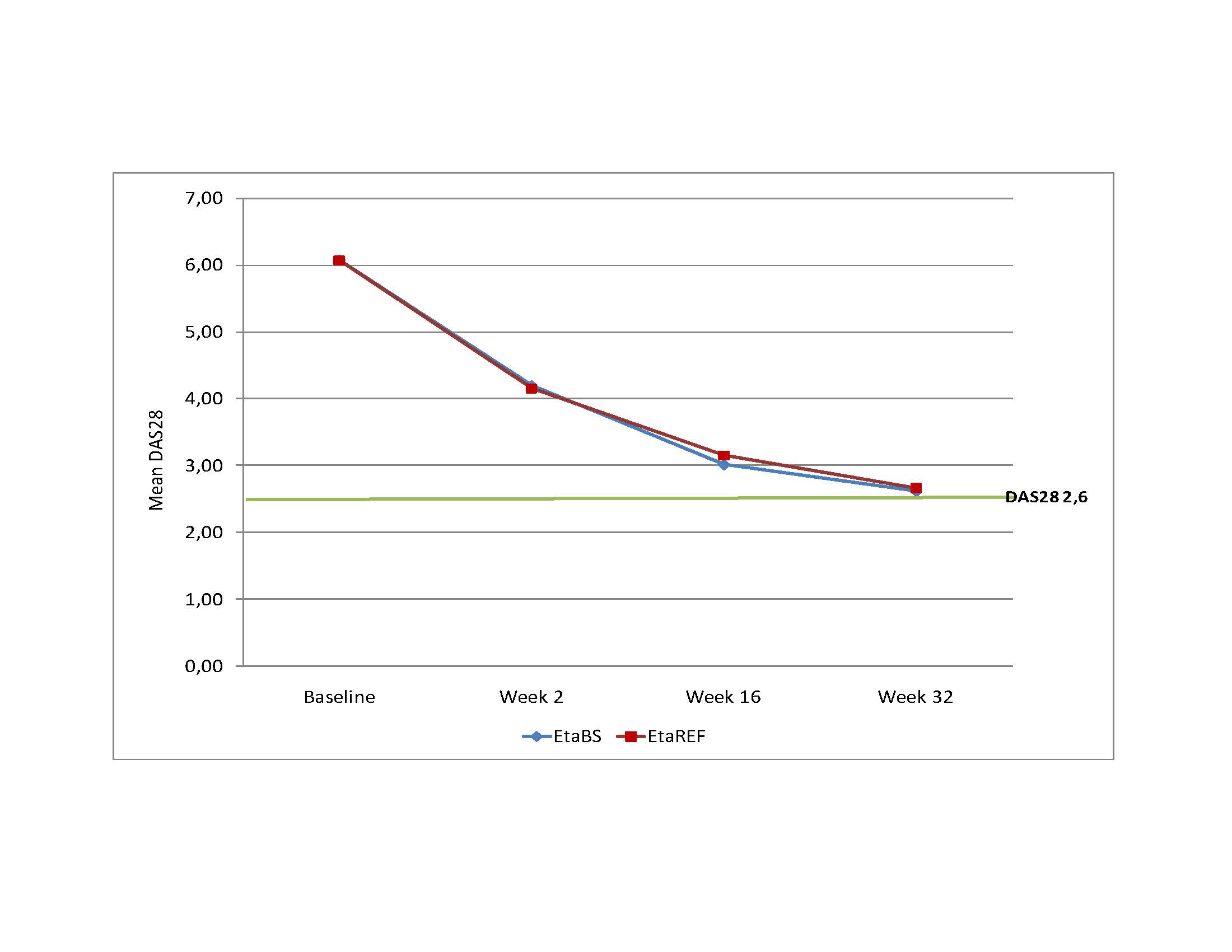
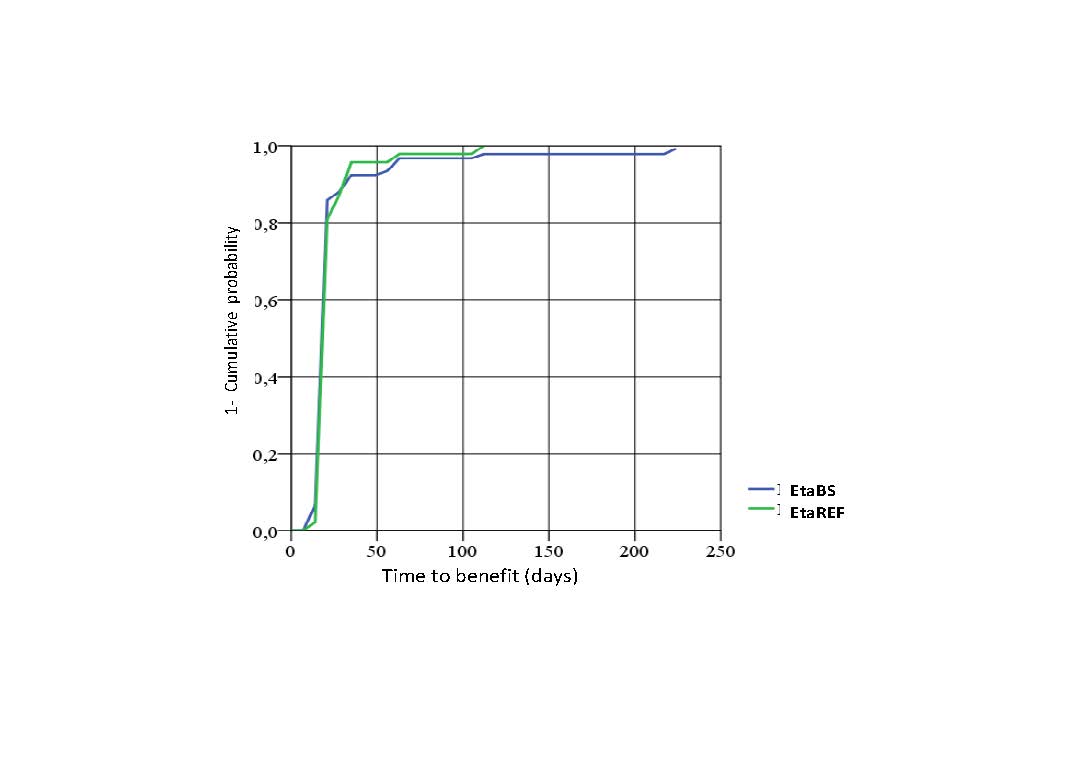
Results: A total of 150 subjects were randomized, 99 were treated with EtaBS and 51 with EtaREF. Five (5) subjects discontinued treatment, 3 subjects treated with EtaBS and 2 treated with EtaREF. Efficacy: Per protocol analysis: 85 (92.4%) subjects treated with EtaBS and 44 (93.6%) subjects treated with EtaREF achieved ACR20 (Difference -1.2% CI 95% -10.1; 7.6%). ITT Analysis: 88 (88.9%) subjects treated with EtaBS and 46 (90.2%) treated with EtaREF achieved ACR20 (Difference -1.3%, CI 95% -11.6; 8.9%). ACR50 was achieved by 68.5% and 59.6% subjects and ACR 70 by 48.9% and 42.6%. Sharp/van der Heijde score without radiological progression was maintained by 98.9% and 97.9% respectively. DAS28 results are observed in Figure 1. Figure 2 shows Time to benefit. Safety: Frequent ADRs were developed by 34 subjects (34.3%) treated with EtaBS and by 19 (38.0%) subjects with EtaREF. The most common reaction was upper respiratory tract infection. Six SAEs were developed by 4 subjects treated with EtaBS (lung cancer, epigastralgia, trombophlebitis of left forearm, spondylodiscitis -probably infective- renal colic and colelithiasis). Three subjects treated with EtaREF developed SAEs (unstable angina, acute pelvic inflammatory disease and carpus surgery). No hepatic alterations were developed. Injection site reactions were developed by 67 subjects (67.7%) treated with EtaBS and 33 subjects (66.0%) with EtaREF. Most frequent local reactions were pain and ecchymosis. No subject discontinued due to these reactions. Immunogenicity: Three subjects, 2 treated with EtaBS and 1 treated with EtaREF developed antibodies by week 32. All of them had good treatment response. Steady State Concentration results are shown in Figure 3.
Conclusion: In this non-inferiority 32 weeks trial, clinical and functional outcomes for EtaBS were not inferior to EtaREF. Both products showed similar safety, immunogenicity and radiographic profiles in patients with moderate to severe active RA on background MTX. EtaBS was granted biosimilarity.
To cite this abstract in AMA style:
Strusberg I, Siri D, Correa M, Scarafia S, Pardo Hidalgo R, Spindler A, Tate P, Venarotti H, Velasco Zamora J, Citera G, Mysler E, Klimovsky E, Federico A, Eizikovits G, Cordeiro L, Lago N. Multicenter, Evaluator-blinded, Randomized, Non-inferiority Study, to Assess the Efficacy, Safety and Immunogenicity of Etanercept Biosimilar (EtaBS) vs. Reference Etanercept (EtaRef) in Combination with Methotrexate for the Treatment of Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/multicenter-evaluator-blinded-randomized-non-inferiority-study-to-assess-the-efficacy-safety-and-immunogenicity-of-etanercept-biosimilar-etabs-vs-reference-etanercept-etaref-in-combination-w/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multicenter-evaluator-blinded-randomized-non-inferiority-study-to-assess-the-efficacy-safety-and-immunogenicity-of-etanercept-biosimilar-etabs-vs-reference-etanercept-etaref-in-combination-w/