Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Sacroiliitis can be detected incidentally on magnetic resonance enterography (MRE) scans in Crohn’s disease (CD) patients, obtained for CD assessment during clinical practice. However, the diagnostic accuracy of MRE-based sacroiliitis has not been studied for clinical relevance and applicability. The purpose of this study was to prospectively enroll CD patients with previously positive MRE-based sacroiliitis and examine them for signs of spondyloarthritis (SpA), undergo standardized MRI of sacroiliac joints (MRI-SIJ) and determine clinical significance of this finding by rheumatological assessment.
Methods: CD patients with evidence of sacroiliitis on routine MRE were prospectively enrolled from the NYU Inflammatory Bowel Disease (IBD) Center. All subjects underwent standard MRI-SIJ at time of follow-up. Data collected included history-related CD, joint symptoms, back pain, 66-68 joint count, enthesitis scores and Harvey Bradshaw Index (HBI, a measure of CD activity). Use of tumor necrosis factor-alpha inhibitors (TNFi) and non-TNFi therapies were recorded at the time of MRE and MRI. MRI-SIJs were read by a blinded board-certified musculoskeletal radiologist. MRI was considered positive for sacroiliitis based on radiologist’s global impression, Assessment of SpondyloArthritis International Society (ASAS) positivity and presence of structural lesions.
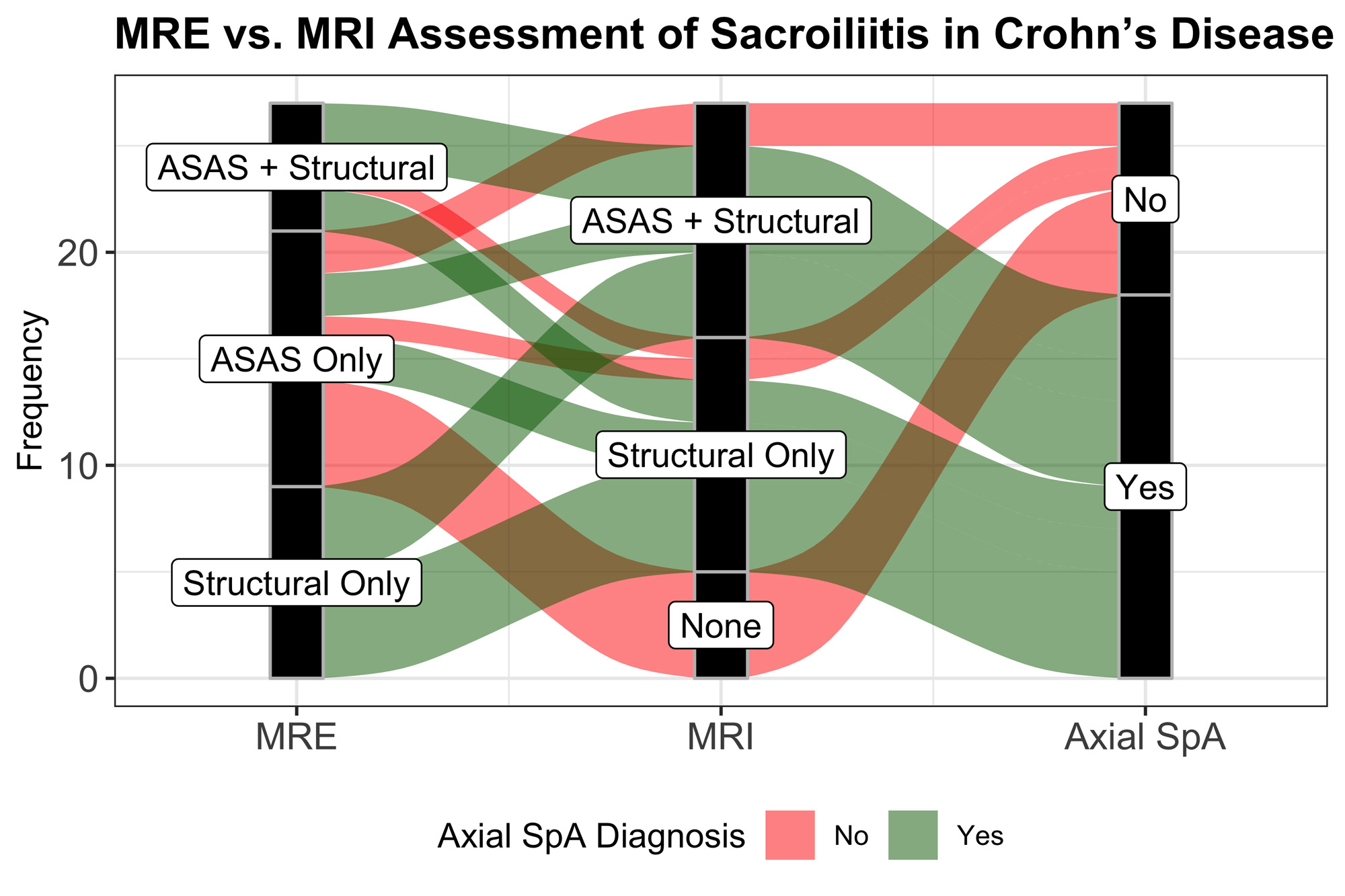
Results: 27 CD subjects with evidence of sacroiliitis on MRE were enrolled. 59.3% (n=16) were female, 88.9% (n=24) were white with a median age of 30 years [IQR: 27 – 43.5]. The median duration of CD was 3.2 years [IQR: 1.06 – 10.1] at the time MRE with a median HBI score of 4 [IQR: 1.5 – 7] consistent with mild CD activity. 37% of subjects were on TNFi and 14.8% were on non-TNFi therapy at the time of MRE. 66.6% (n=18) fulfilled ASAS positivity and 55.5% (n=15) with at least 1 structural lesion on MRE. The median duration between MRE and follow-up MRI was 2.13 years [IQR: 0.57 – 3.42]. 81.5% (n=22) of MRE-positive patients had evidence of sacroiliitis on the follow-up MRI, with 40.7% (n=11) fulfilling ASAS positivity. 23 subjects had back pain, and 15 subjects had inflammatory back pain (IBP) at the time of MRI. Of those with IBP, 93.3% (n=14) had MRI evidence of sacroiliitis. 66.7% (n=18) were determined to have Axial SpA (axSpA) by the assessing rheumatologist at the time of MRI. Median HBI was 3 [IQR: 2-6] at the time of MRI, suggesting remission. 48.1% and 33.3% of CD subjects were on TNFi and non-TNFi, respectively at the time of MRI. CD patients with structural lesions on the initial MRE were significantly more likely to have axSpA diagnosis on rheumatological assessment compared to those without (93.3% vs. 33.3%, p=0.003) (Figure 1).
Conclusion: Sequentially examined positive MRE scans in CD patients, obtained for standard of care, revealed a surprisingly high percentage of MRI-confirmed cases of sacroiliitis, many associated with clinical findings of axSpA. This study emphasizes the importance of MRE as a valuable screening tool to detect “hidden” sacroiliitis in CD patients, prompting rheumatological evaluation, which can impact therapeutic decision-making since only some of the currently available therapies for CD are effective in axSpA.
To cite this abstract in AMA style:
Malik F, Gyftopoulos S, Axelrad J, Chang S, Hong S, McAllister M, Olateru Olagbegi M, Alaia E, Walter W, RA SLE Network A, Hudesman D, Scher J. MRI Confirms MRE Evidence of Sacroiliitis in Many with Crohn’s Disease: A Prospective Study for the Screening of axSpA in High-risk Populations [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/mri-confirms-mre-evidence-of-sacroiliitis-in-many-with-crohns-disease-a-prospective-study-for-the-screening-of-axspa-in-high-risk-populations/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mri-confirms-mre-evidence-of-sacroiliitis-in-many-with-crohns-disease-a-prospective-study-for-the-screening-of-axspa-in-high-risk-populations/