Session Information
Date: Saturday, November 6, 2021
Title: Epidemiology & Public Health Poster I: COVID-19 & Vaccination (0084–0117)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Patients with rheumatic and musculoskeletal diseases (RMDs) remain at an increased risk of morbidity and mortality from vaccine preventable infections, often as a result of disease activity, comorbidities, and immunosuppressive medication.1 Despite increased risk, vaccine uncertainty and hesitancy remain ongoing challenges among this population and continue to be observed during the COVID-19 pandemic.2 Even as COVID-19 vaccinations become more accessible, relatively low acceptance of the vaccine among the general population may negatively impact RMD populations, who would benefit from increased protection against COVID-19 illness.3
Methods: We conducted a web-based follow-up survey of spondyloarthritis (SpA) patients in the U.S. and Canada who had previously participated in a Spondylitis Association of America-sponsored COVID-19 survey. Fifteen multiple-choice questions were designed to capture information related to COVID-19 vaccine acceptance, attitudes, medication modification, perceived impact of vaccination on disease activity, past and present vaccine hesitancy or refusal, and COVID-19 vaccine information sources among SpA patients. Beginning April 1st 2021, participants were prompted to complete the follow-up questions via an email blast and social media posts.
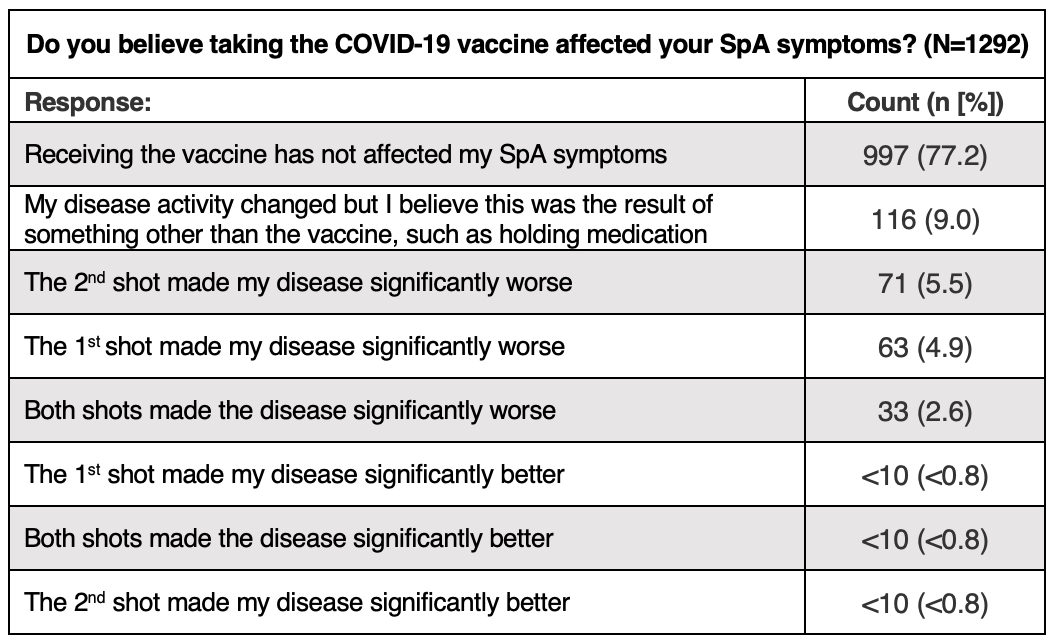
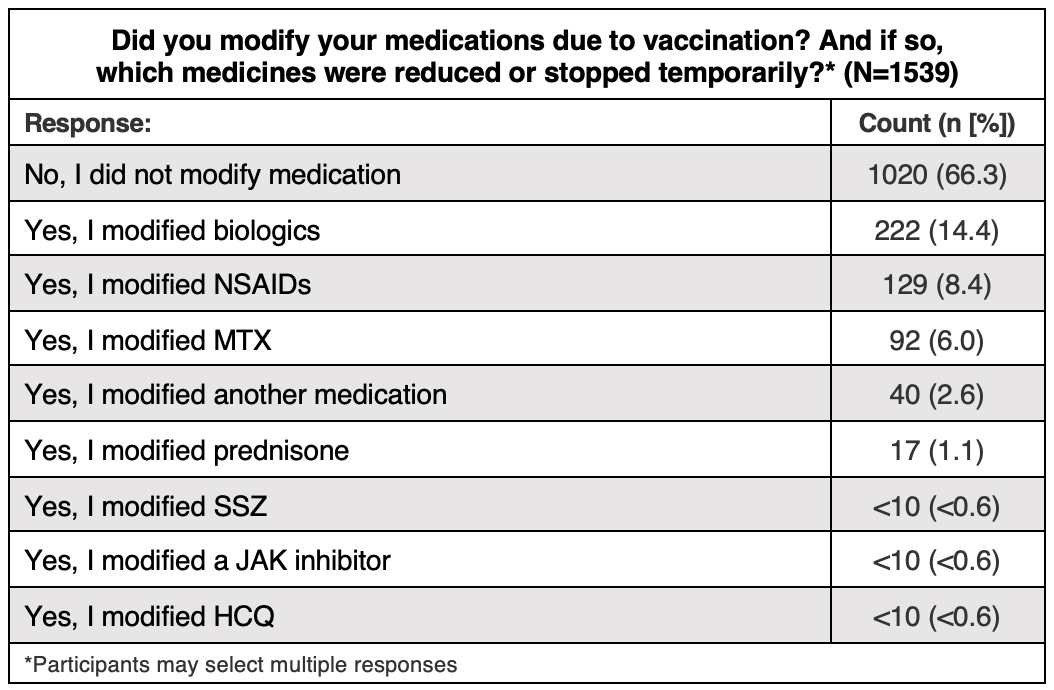
Results: Between April 1st and April 27th, 1745 self-reported SpA participants responded to the survey. Overall, 1430 (81.9%) indicated they had received at least one dose of a COVID-19 vaccine and 10.1% planned to be vaccinated in the future. Fifty-one (2.9%) were undecided about receiving a COVID-19 vaccination and only 88 (5%) did not intend to receive the vaccine in the future. Among the 1539 responses from vaccinated participants (multiple selections possible), 1020 (66.3%) did not modify their SpA treatment due to vaccination, 222 (14.4%) modified a biologic, 129 (8.4%) modified an NSAID, 92 (6%) modified MTX, and 73 (4.9%) modified another medication. Among 1292 vaccinated participants, 997 (77.2%) indicated the COVID-19 vaccine had no effect on their SpA symptoms, 167 (12.9%) reported worsening symptoms after the first, second, or both vaccines, and 116 (9%) indicated a change in disease activity that they attributed to another factor, such as modified medication. Among 194 unvaccinated participant responses (multiple selections possible), 61 (31.4%) indicated fear of potential vaccine side effects as their main reason to decline, 39 (20.1%) indicated that the vaccine wasn’t mandated and it was their right to decline, and 36 (18.6%) indicated fear of worsened disease symptoms.
Conclusion: Willingness to receive the COVID-19 vaccine was high and uncertainty and hesitancy were low among survey participants; over half of respondents were eager to be vaccinated. Nearly 80% of vaccinated participants indicated that the vaccine had no effect on their SpA symptoms, and only 1/3 modified their SpA treatment due to vaccination. These findings highlight the lower impact of vaccination on SpA disease activity, and may encourage other hesitant or unsure SpA patients concerned about increased disease activity to get vaccinated against SARS-CoV-2.
To cite this abstract in AMA style:
Higgins R, Hamilton H, Weisman M, Reveille J, Ogle K, Shafer C, Aslanyan E, Howard R, Choi D, Rosenbaum J, Winthrop K. Most Patients with Spondylitis Accept COVID-19 Vaccination and Few Experience Disease Exacerbation After Immunization [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/most-patients-with-spondylitis-accept-covid-19-vaccination-and-few-experience-disease-exacerbation-after-immunization/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/most-patients-with-spondylitis-accept-covid-19-vaccination-and-few-experience-disease-exacerbation-after-immunization/