Session Information
Date: Tuesday, November 15, 2016
Title: Epidemiology and Public Health II: Obesity, Cancer and Mortality
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Pulmonary involvement, including interstitial lung disease (ILD), is common in patients with rheumatoid arthritis (RA) and is associated with increased mortality. Early reports suggested that TNFα inhibitors (TNFi) may be linked to the development, or exacerbation, of ILD, and in response, in 2005 the British Society for Rheumatology advised caution in their use for patients with RA-ILD. In contrast, no comparable recommendations have been provided for rituximab (RTX), which is often used where contra-indications for TNFi exist. This study aimed to examine 5-year mortality in patients with RA-ILD starting either RTX or TNFi as their first biologic therapy for RA.
Methods: Participants in the British Society for Rheumatology Biologics Register for RA with clinician reported RA-ILD at baseline starting either TNFi or RTX as their first biologic for RA were included in this analysis. Date and cause of death were captured on regular study follow-up forms and through linkage with the UK National Death Register. Death rates, per 1000 person years (pyrs) were calculated (95% CI); censoring occurred at death, 12/6/2015, or 5 years following first registration, whichever came first. The frequency with which ILD was mentioned on death certificates was examined. Kaplan-Meier survival curves were generated, with risk comparisons made between the RTX and TNFi cohorts using Cox regression using an ever exposed model, adjusted for potential confounders. Eligibility of confounders was determined by clinically relevant justification or statistical significance (p<0.05), after adjustment for treatment effects.
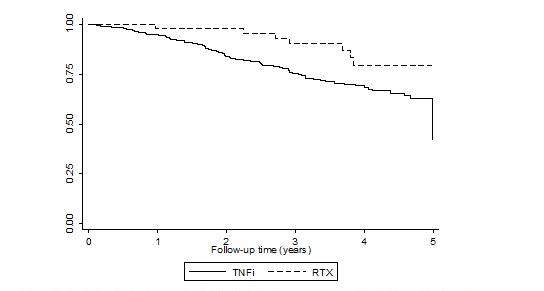
Results: Of 353 eligible RA participants with RA-ILD, 310 were treated with TNFi (all recruited before 2008) and 43 were treated with RTX patients (all recruited after 2008). During the first 5 years of follow-up, there were 76 deaths in 804.9 pyrs observed in the TNFi cohort and 8 deaths in 156.7 pyrs in the RTX cohort; death rates were 94.4 (74.4-118.1) and 51.0 (22.0-100.5) per 1000 pyrs, respectively. ILD was recorded on 36.5% of the 74 available death certificates from the TNFi cohort and all of the three certificates available in RTX cohort. The unadjusted mortality risk in the RTX treated patients was numerically half that in the TNFi treated patients, although this was not statistically significant (HR 0.51, 95%CI 0.25-1.06; Figure 1). Adjustment for baseline age, sex, disability, disease activity, and disease duration made little difference to the estimate (0.50, 0.23-1.08).
Figure 1 – Kaplan-Meier survival curves for death following exposure to TNFi or RTX over the first 5 years following therapy commencement, within an intention to treat analysis.
Conclusion: Patients who were selected to receive RTX as their first biologic for RA appeared to have better long-term survival compared to patients who had received TNFi, although this difference was not statistically significant. Absence of information on severity or subtype of ILD prevents drawing conclusions regarding the relative safety of these 2 therapies for RA-ILD and more detailed analysis in a larger dataset should be undertaken.
To cite this abstract in AMA style:
Druce K, Iqbal K, Watson K, Symmons DPM, Hyrich KL, Kelly C. Mortality in Patients with Rheumatoid Arthritis and Interstitial Lung Disease Treated with First Line TNFi or Rituximab Therapies [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/mortality-in-patients-with-rheumatoid-arthritis-and-interstitial-lung-disease-treated-with-first-line-tnfi-or-rituximab-therapies/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mortality-in-patients-with-rheumatoid-arthritis-and-interstitial-lung-disease-treated-with-first-line-tnfi-or-rituximab-therapies/