Session Information
Date: Monday, October 27, 2025
Title: Plenary II (0849–0854)
Session Type: Plenary Session
Session Time: 8:00AM-8:15AM
Background/Purpose: Glucagon-like-peptide-1 receptor agonists (GLP-1 RAs) are the novel drug class that has gained widespread attention for its cardiovascular, renal and weight loss benefits in patients with type 2 diabetes mellitus. Available evidence has illustrated that weight loss, through surgical or behavioral modification, is associated with improvements in disease activity in patients with obesity and RA, PsA, or psoriasis.
Methods: This is a nationwide, retrospective study. We used the global, multicenter research network (TrinetX) database from 83 large healthcare organizations across multiple countries. We included patients older than 18 years old with a diagnosis of psoriatic arthritis, for the last 10 years (between January 2015 and December 2024), using the International Classification of Disease-10 codes. We compared psoriatic arthritis patients on GLP-1 RAs (Semaglutide, Liraglutide, Exenatide, Lixisenatide), with psoriatic arthritis patients not on GLP-1 RAs. We used 1:1 propensity score matching that accounted for demographics variables, diagnoses, and medication use. Index event was defined as simultaneous appearance of psoriatic arthritis and GLP-1 RA use in patients’ charts. Outcome of interest was the incidence of major adverse cardiovascular effects and overall mortality. Patients displaying these outcomes before the index event were excluded from the analysis. The exposure for the outcome was any time after the diagnosis of psoriatic arthritis during the time window. Risk ratios with 95% confidence intervals were calculated for each outcome. A two-sided p-value less than 0.05 was considered statistically significant.
Results: We identified 4,104 psoriatic arthritis patients taking GLP-1 receptor agonists (GLP-1 RAs) and 86,432 psoriatic arthritis patients not taking GLP-1 RAs. At baseline, the mean age at diagnosis was 55.4 ± 11.5 years for those on GLP-1 RAs and 52.6 ± 15.1 years for those not on GLP-1 RAs. Female patients were more prevalent in GLP-1 RA group, on the contrary male patients were predominant in no GLP-1 RAs. White patients made up the largest racial group in both subgroups (80.9% for GLP-1 RA users and 73.5% for non-users). After propensity score matching, patients on GLP-1 RAs showed lower risk of developing major cardiovascular adverse events and had reduced mortality compared to those not using GLP-1 RAs (Table 2).
Conclusion: Psoriatic arthritis patients receiving GLP-1 receptor agonists have lower risk of developing major adverse cardiac events, and have reduced mortality compared to psoriatic arthritis patients not taking GLP-1 receptor agonists. GLP-1 receptor agonists may be a promising adjunct in the management of patients with inflammatory arthritis, who have comorbidities including obesity and/or type 2 diabetes mellitus. Further research is warranted to better understand the mechanisms underlying these outcomes and to confirm the long-term benefits of GLP-1 RAs.
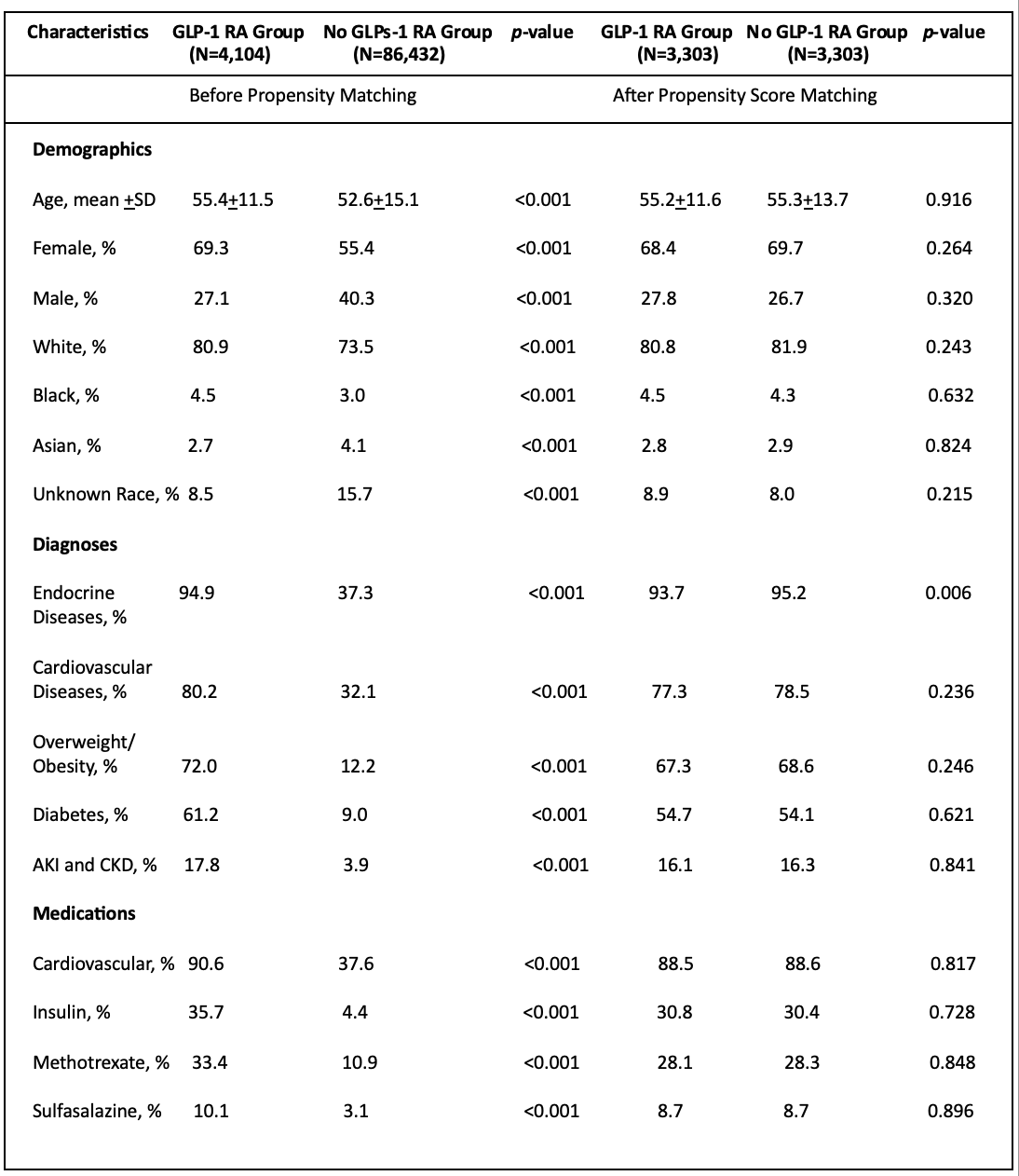 Table 1. Baseline Patient Characteristics of Study Groups, before and after Propensity Score Matching
Table 1. Baseline Patient Characteristics of Study Groups, before and after Propensity Score Matching
.jpg) Table 1. Baseline Patient Characteristics of Study Groups, before and after Propensity Score Matching (Ctd.)
Table 1. Baseline Patient Characteristics of Study Groups, before and after Propensity Score Matching (Ctd.)
.jpg) Table 2. Risk of major adverse cardiac events in psoriatic arthritis patients on GLP-1 RA therapy,
Table 2. Risk of major adverse cardiac events in psoriatic arthritis patients on GLP-1 RA therapy,
versus not on GLP-1 RA therapy
To cite this abstract in AMA style:
Tsibadze N, Tskhakaia I, Tan I. Mortality and Major Adverse Cardiac Events (MACE) with GLP-1 Receptor Agonists in Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/mortality-and-major-adverse-cardiac-events-mace-with-glp-1-receptor-agonists-in-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mortality-and-major-adverse-cardiac-events-mace-with-glp-1-receptor-agonists-in-psoriatic-arthritis/
