Session Information
Session Type: Late-Breaking Abstracts
Background/Purpose:
Long-term use of glucocorticoids (GC) is problematic for patients with increased risk for vertebral fractures, especially those with rheumatoid arthritis (RA). Thus, prevention of GC-induced osteoporosis (GIOP) with bisphosphonates has now become an important strategy for reducing morbidity in RA patients. Ibandronate is indicated for the treatment and prevention of osteoporosis in postmenopausal women. However, evidence for ibandronate in GIOP prevention has been insufficient to date. This aim of this study was to investigate the efficacy of monthly oral ibandronate in RA women with reduced bone mineral density receiving long-term GCs.
Methods:
Female RA patients meeting the 1987 ACR classification criteria were enrolled in this 48-week double-blinded randomized placebo-controlled trial (clinicaltrials.gov NCT01287533). Patients that had taken GC (prednisolone-equivalent dose of 5 mg or higher) for 3 or more consecutive months and fulfilling L1-4 T-score of < -1.0 and -2.5 or higher by dual-energy X-ray absorptiometry were enrolled in this study. Patients were divided into 2 groups: 150 mg ibandronate versus placebo po every 4 weeks. Both groups were provided with daily 1500 mg of calcium carbonate and cholecalciferol of 400 IU. The primary end point was to compare the % changes of the L1-4 T-score at 48 weeks compared with baseline.
Results:
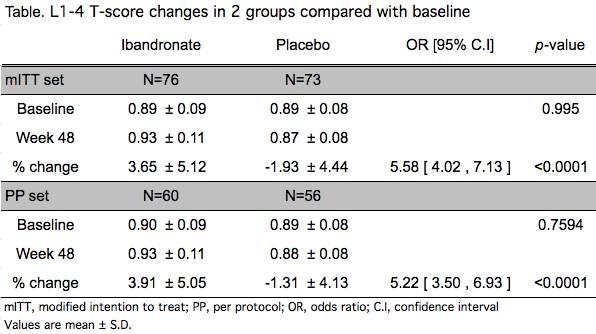
Two hundred eleven patients from 13 centers nationwide participated in this study. One hundred sixty seven patients were randomized. The mean age of patients in the ibandronate and placebo group were 54.5 and 55.1 years, respectively. Baseline characteristics were not dissimilar between the 2 groups. The result of the primary endpoint is shown below (table). Serum C-terminal telopeptide was significantly reduced at 48 weeks in the ibandronate group compared with placebo (-53.1 ± 46.2 % versus 4.23 ± 58.3 %, p<0.0001, mIIT set). There was no incident of fragility fracture in both groups during the study period. Safety profiles including adverse events were comparable between the 2 groups.
Conclusion:
Monthly oral ibandronate for 48 weeks was effective in reducing bone loss in RA women on long-term glucocorticoids. Longer follow-up studies would be needed to investigate the effect of ibandronate on fracture prevention in such clinical settings.
Disclosure:
K. Shin,
None;
S. H. Park,
None;
W. Park,
None;
H. J. Baek,
None;
Y. J. Lee,
None;
S. W. Kang,
None;
J. Y. Choe,
None;
W. H. Yoo,
None;
Y. B. Park,
None;
J. S. Song,
None;
B. Yoo,
None;
D. H. Yoo,
None;
Y. W. Song,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/monthly-ibandronate-reduces-bone-loss-in-osteopenic-women-with-rheumatoid-arthritis-receiving-long-term-glucocorticoids-a-48-week-double-blinded-randomized-placebo-controlled-investigator-initiated-t/