Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Methotrexate (MTX) and Leflunomide (LEF) are widely prescribed to treat rheumatologic and other diseases. Each has the potential to cause liver injury in some patients. Current guidelines recommend regular monitoring of either ALT or AST every 2-3 months even in patients on a stable, long-term dose. Adherence to monitoring guidelines has not been reported for a large, community-based population. As part of a quality improvement project, we assessed adherence to monitoring guidelines as well as practices used by rheumatologists to assure monitoring.
Methods: First, we queried the clinical databases of Kaiser Permanente Northern California to identify all patients who had received 2 or more prescriptions of MTX or LEF, with at least 1 prescription dispensed in the previous 6 months. Adherence to guidelines was defined as having a result for ALT or AST in the 90 days prior to data collection. Second, to assess knowledge of guidelines, as well as clinical prescribing and monitoring practices, we surveyed 40 rheumatologists.
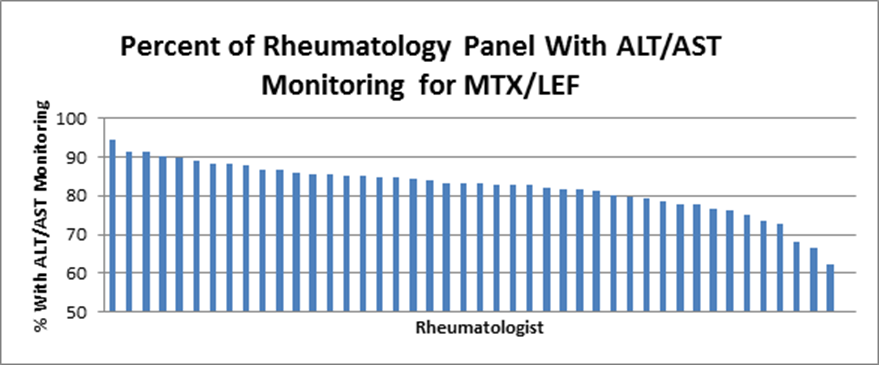
Results: We identified 8,276 Internal Medicine patients on MTX/LEF. Among the rheumatologists, the rate of adherence ranged from 62.3 to 94.6% (weighted mean, 82.3%) (Figure). Survey of the rheumatologists (87.5% response) revealed that all were knowledgeable of guidelines, all prescribed ≤3 month supply of medication, 94% used standing laboratory orders, and 71% did not refill prescriptions in patients without an ALT or AST in the prior 3 months.
Conclusion: Despite use of an electronic health record, knowledge of monitoring guidelines, and efforts to assure adherence, we observed significant variation in monitoring liver toxicity in a community rheumatology practice. Our results underscore the need for more effective tools and workflows that integrate prescribing with monitoring. Such solutions, implemented at the system level, would have wide applicability for drugs used across a range of diseases and specialty areas.
Disclosure:
R. Goldfien,
None;
L. Herrinton,
Medimmune,
2.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/monitoring-methotrexate-and-leflunomide-treatment-for-liver-toxicity-the-kaiser-permanente-experience/