Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The best treatment option after a patient has failed a first TNFi is still unknown. Therefore the objective of this randomized open label study is to compare the effectiveness of abatacept, rituximab or another TNFi after failing a first TNFi, with the DAS28 as outcome measure, in patients with Rheumatoid Arthritis.
Methods: The inclusion criteria for this pragmatic randomized trial within the DREAM cohort were: failing a first TNFi, a DAS28 > 3.2, not treated before with abatacept or rituximab and no contraindications. Patients were randomized to abatacept, rituximab or TNFi treatment. The DAS28 was compared between the three groups with ANOVA, 6 and 12 month after randomization.
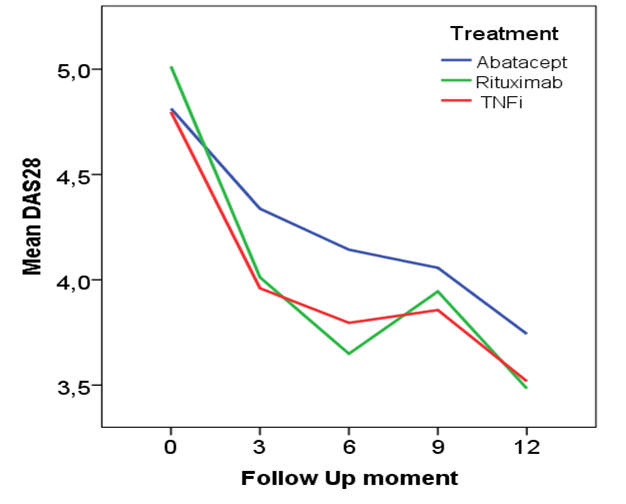
Results: 143 patients were randomized into one of the treatments (mean age 56.5 yrs, 76.5% female, median disease duration 5.85 yrs, 63.6% rheumatoid factor positive, mean DAS28 of 4.9). The mean DAS28 (+95% Confidence Interval) after 6 and 12 months respectively was 4.1 (3.7-4.6) and 3.7 (3.2-4.2) for abatacept, 3.6 (3.2-4.1) and 3.5 (3.0-3.9) for rituximab and 3.8 (3.3-4.3) and 3.5 (2.9-4.1) for TNFi, see figure. These were not statistically significant. Remission (DAS28<2.6) was attained in 10.7% and 12.0% in the abatacept group, 24.3% and 27.3% in the rituximab group and 12.9% and 24.1% in the TNFi group at 6 and 12 month respectively.
Conclusion: The data do not reveal a significant difference in effectiveness, measured with the DAS28, between the three different biological. Therefore other reasons than DAS28 status at 6 and 12 months might play a more important role in the choice of a second biological like long term stability of response, side effects, costs or patients preferences for route of administration of treatments.
Disclosure:
S. H. M. Manders,
None;
W. Kievit,
None;
H. L. M. Brus,
None;
H. J. Bernelot Moens,
None;
A. Hartkamp,
None;
R. Bos,
None;
E. Brouwer,
None;
H. Visser,
None;
H. E. Vonkeman,
None;
R. Westhovens,
BMS,
8,
Janssen; Galapagos,
9,
Roche Pharmaceuticals,
2;
M. A. F. J. van de Laar,
None;
P. L. Van Riel,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mode-of-action-change-not-necessary-after-failing-the-first-tumornecrosisfactor-inhibitor-preliminary-results-of-a-randomized-controlled-trial/