Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The German pregnancy register Rhekiss is designed as a nationwide, web-based longitudinal observational register established in 2015. The register follows women with inflammatory rheumatic disease prospectively from child wish through pregnancy until two years postpartum. During the observation, information on clinical and laboratory parameters, disease activity, comorbidities, flares, drug treatment, and (adverse) pregnancy outcomes are documented in pre-specified intervals. Physicians and patients (pts) report data for the same time periods via separated accounts and questionnaires (Qs) into a web-based application on personal computers, laptops, and notebooks. Due to the increasing use of mobile devices, a responsive App as a further documentation option and for facilitated patient-reported data entry was developed. We examined whether pts use an App for data entry in the pregnancy register.
Methods: The Rhekiss-App is available in German and can be downloaded for self-reported data retrieval since 08/2017 from App stores (Android and iOS). For the current analysis, Rhekiss register data were used from the time period October 2018 until April 2019 to ensure valid data on the applications modes.
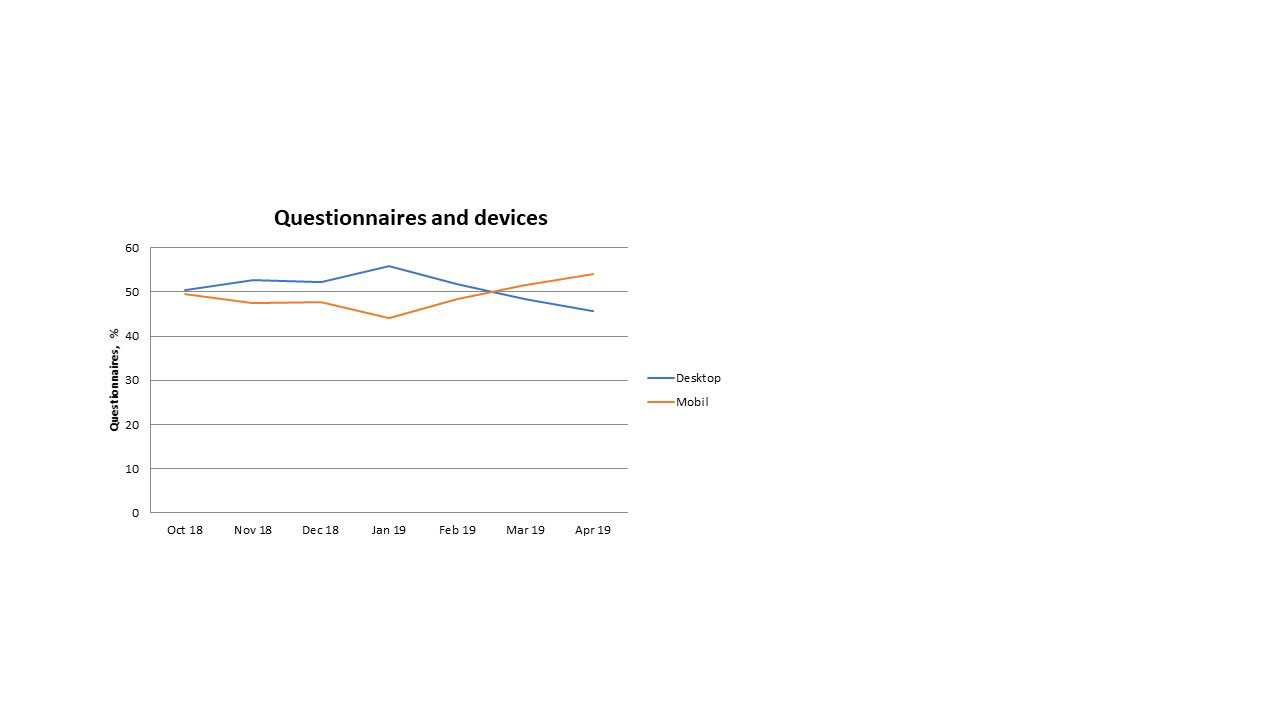
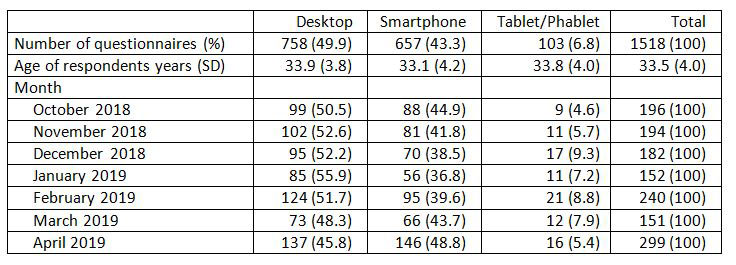
Results: Within the analyzed period Rhekiss expected answers from 2207 Qs of 889 pts. Overall, 71.2% of the Qs were filled in. 49.9% of the Qs were sent from a browser installed on a desktop computer, ranging from 56% (January 2019) to 46% (April 2019), see table 1. Desktop users were on average 34±3.8 years old; the proportion of desktop users was highest in women after delivery (52%). The App installed on mobile devices was used for data entry of 760 Qs (50.1%): Smartphone use ranged between 37% and 49%, tablet use was less frequent (up to 9%), and phablet use was neglectable, see table 1. Women with child wish had the highest smartphone App use (51%). Figure 1 depicts the progress of desktop and mobile documented Qs.
Conclusion: The responsive App is a useful additional tool for data collection in Rhekiss. It was used for data acquisition from approximately 50% of the questionnaires. While the use of the App on a smartphone was of high interest, the use of tablets/phablets for data collection seems negligible. Apart from desktop/browser developments, technological adoptions within observational cohorts and/or future (joined) registries need to take smartphone requirements and developments into account, especially when patient-reported data in young, mobile patients are collected.
Acknowledgement: The register’s mobile App was implemented with software development from the IT partners Serrala, Berlin (Germany) and Questback, Köln (Germany)
To cite this abstract in AMA style:
Richter J, Bungartz C, Weiß A, Fischer-Betz R, Zink A, Schneider M, Strangfeld A. Mobile Responsive App – Useful Additional Tool for Data Collection in the German Pregnancy Register Rhekiss? [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/mobile-responsive-app-useful-additional-tool-for-data-collection-in-the-german-pregnancy-register-rhekiss/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mobile-responsive-app-useful-additional-tool-for-data-collection-in-the-german-pregnancy-register-rhekiss/