Session Information
Date: Wednesday, October 24, 2018
Title: 6W010 ACR Abstract: SLE–Clinical V: Biomarkers, Criteria, & Outcomes (2928–2933)
Session Type: ACR Concurrent Abstract Session
Session Time: 9:00AM-10:30AM
Missing data due to drop-out and loss to follow-up is a common problem in SLE trials. The usual approaches for handling this issue include analyzing only subjects with complete data (complete case analysis; CC), last observation carried forward (LOCF), or imputing non-responses for missing outcomes (non-responder imputation; NRI). However, the validity of these methods depends on strong assumptions about the missing data mechanism. Multiple imputation (MI) is a flexible model-based technique that accounts for uncertainty in the imputation process by generating several possible values for the missing data, resulting in multiple complete data sets. These are analyzed separately and results are combined. MI is being used more widely in different disease settings but has not been applied to analyze the primary outcome in a SLE trial. We explored the use of MI to address missing data in the composite outcome, SLE Responder Index (SRI)-5, using data from patients assigned to standard of care (SoC) in a 52-week trial.
Methods:
Data on 279 SLE patients randomized to SoC for 52 weeks who were receiving mycophenolate mofetil (MMF), azathioprine, or methotrexate at entry were obtained from the Lupus Foundation of America-Collective Data Analysis Initiative database. Multiple imputation using chained equations was applied to handle missing data in an analysis to evaluate differences in SRI-5 response rates at 52-weeks between patients treated with MMF versus other immunosuppressants (non-MMF). Three different imputation models were considered that included various combinations of longitudinal measures of disease activity (both composite and individual measures) and patient characteristics to impute the missing outcomes. Results were compared to estimates from the CC, LOCF, and NRI methods.
Results:
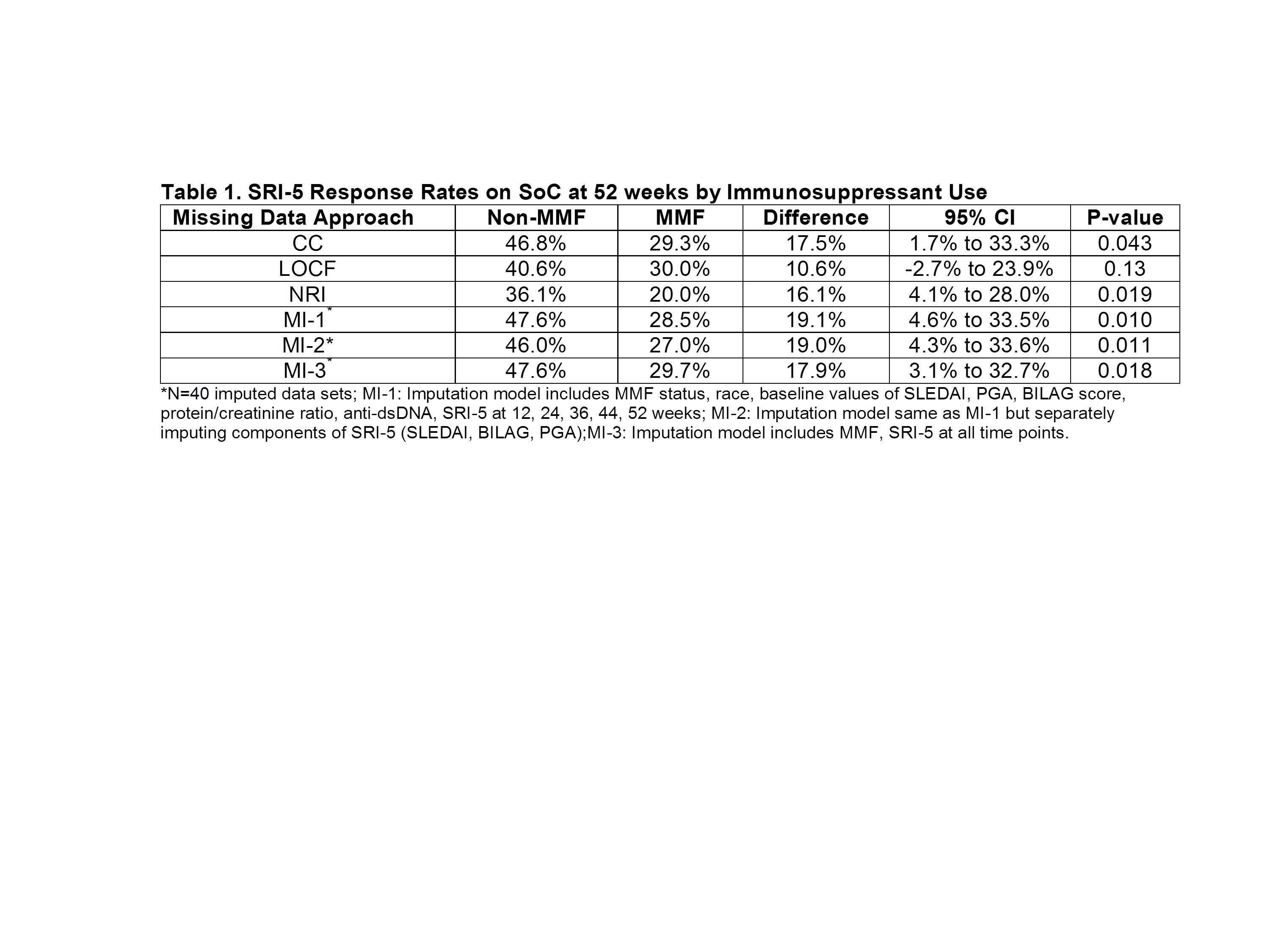
Missing data rates were 32% in the MMF and 23% in the non-MMF groups. As expected, the NRI missing data approach yielded the lowest response rates (Table 1). The smallest and least significant estimates of between group differences were observed with LOCF. The precision of the estimated difference, as measured by the width of the confidence interval, was lowest with the CC method because of the reduced sample size. Group differences were magnified with all three MI models compared to results of other methods. Imputing SRI-5 directly (MI-1) versus the individual components (MI-2) yielded nearly identical results.
Conclusion:
Given the limitations of conventional approaches for handling missing data, the MI method should also be considered in SLE trials. However, results can vary depending on the imputation model that is used, and the assumptions required for validity of this and other missing data methods must be justified. Sensitivity analysis using different approaches is important to demonstrate robustness of results especially when missing data rates are non-negligible.
To cite this abstract in AMA style:
Kim M, Merrill JT, Kalunian KC, Hanrahan L, Izmirly PM. Missing Outcomes in SLE Clinical Trials: Impact on Estimating Treatment Effects [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/missing-outcomes-in-sle-clinical-trials-impact-on-estimating-treatment-effects/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/missing-outcomes-in-sle-clinical-trials-impact-on-estimating-treatment-effects/