Session Information
Date: Monday, October 27, 2025
Session Type: Abstract Session
Session Time: 10:00AM-10:15AM
Background/Purpose: This trial aimed to compare the efficacy and safety of methotrexate (MTX) vs. tocilizumab (TCZ), in combination with glucocorticoid (GC), in patients with giant cell arteritis (GCA).
Methods: METOGiA is an open label randomized controlled multicenter trial, with a non-inferiority design. Results from the first 78-week period are reported here, in which patients received either TCZ 162 mg/week subcutaneously (SC) or MTX 0.3 mg/Kg/week SC (without exceeding 20 mg/week) during 52 weeks in combination with a GC taper regimen (42 weeks for new onset GCA, 36 weeks for relapsing GCA). Eligible patients were aged ≥50 years, diagnosed with new-onset or relapsing GCA and had active GCA within 6 weeks before randomization. The primary endpoint was collected at week 78 (W78) and was the percentage of patients alive, without relapse after initial remission or deviation from the GC taper regimen from inclusion to W78. Secondary endpoints included the percentage of patients alive, without relapse after initial remission or deviation from the GC taper regimen from inclusion to W52; the percentages of patients in remission without prednisone or with prednisone ≤5mg/day at W52 and W78; the cumulative dose of prednisone at W52 and W78; and adverse events. Remission was defined as the absence of symptoms attributable to GCA and CRP≤10 mg/L. Relapse was defined as the recurrence of symptoms attributable to active GCA, whatever the value of CRP. Relapses were confirmed by an adjudication committee composed of 3 experts not participating to the study and blinded to the assigned study arm, CRP, ESR, fibrinogen and FBS values.
Results: A total of 230 patients were randomized in 39 French centers, among them 9 withdraw their consent and 3 were lost of follow up before reaching W78. Finally, 218 patients (TCZ, n=110; MTX, n=108) were analyzed in the intention-to-treat (ITT) population. GCA characteristics were balanced between groups, with 74% and 26% of patients having new-onset and relapsing GCA (TCZ: 77% and 23%; MTX: 71% and 29%), respectively. At W78, the primary endpoint was reached in 37% of MTX vs. 46% of TCZ patients, resulting in a 9% difference (95% CI: [-4%, 22%]), which did not meet the predefined non-inferiority margin of 20%. Thus, in the ITT population, MTX was not non-inferior to TCZ (P=0.054). However, TCZ was not superior to MTX at W78 (P=0.16). Results for secondary endpoints are detailed in Table 1. Relapse-free survival curves are depicted in Figure 1. By W78, 15 MTX-related SAEs occurred in 14 patients (8 infections, including 5 Pneumocystis infections, one of which was fatal) and 13 TCZ-related SAEs occurred in 11 patients (6 neutropenia, 3 infections). Seven deaths were reported at W78 (TCZ, n=1; MTX, n=6).
Conclusion: In the ITT population, MTX was not non-inferior to TCZ regarding the primary endpoint at W78, but was non-inferior for the remission rate with or without low-dose prednisone. At W52, results suggest that TCZ is more effective than MTX in maintaining remission and preventing relapse. These results must be confirmed by the per-protocol analysis (ongoing).ClinicalTrials.gov identifier: NCT03892785
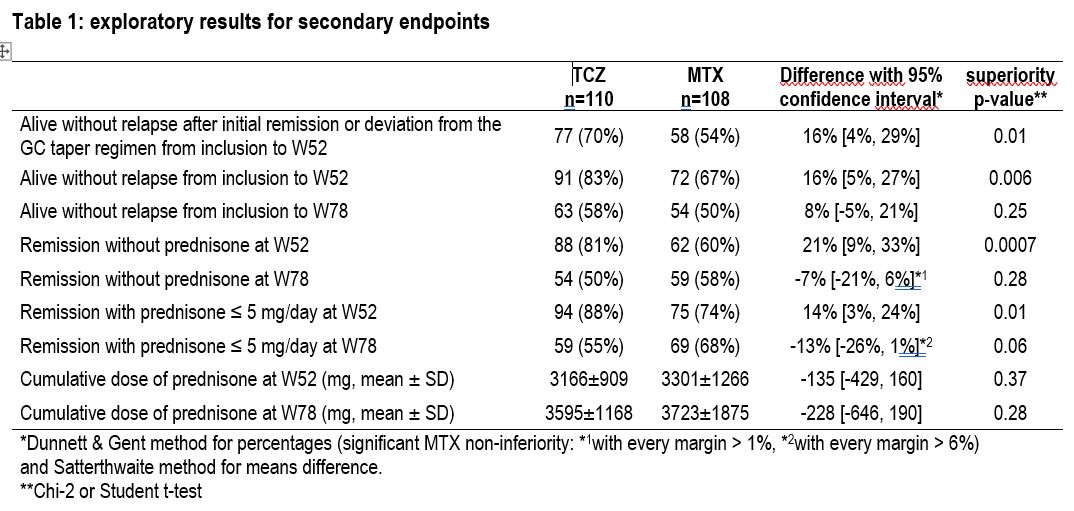 Table 1: exploratory results for secondary endpoints
Table 1: exploratory results for secondary endpoints
.jpg) Figure 1: Relapse free survival (Kaplan-Meier estimates with number of subjects at risk)
Figure 1: Relapse free survival (Kaplan-Meier estimates with number of subjects at risk)
To cite this abstract in AMA style:
Samson M, Bourredjem A, Outh R, Grobost V, Lifermann F, DIREZ G, Ribeiro E, Oziol E, Devauchelle V, Ebbo M, Limal N, Paule R, Bienvenu B, Poulet A, Miranda S, Benhamou Y, Quemeneur T, Dingremont C, Ramon A, Simorre B, Reynaud Q, AGARD C, Espitia O, Mekinian A, Alexandra J, Berthoux E, Perard L, Baudet A, Mesbah R, Lacôte-Delarbre D, Stievenart J, André M, Allain J, Jaussaud R, Magnant J, Servettaz A, De Boysson H, Ruivard M, Terrier B, REGENT A, Cransac A, Fournel I, Devilliers H, Bonnotte B. MEthotrexate versus TOcilizumab for treatment of Giant cell Arteritis (METOGiA trial): a multicenter, randomized, controlled trial. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/methotrexate-versus-tocilizumab-for-treatment-of-giant-cell-arteritis-metogia-trial-a-multicenter-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/methotrexate-versus-tocilizumab-for-treatment-of-giant-cell-arteritis-metogia-trial-a-multicenter-randomized-controlled-trial/
