Session Information
Date: Tuesday, October 23, 2018
Title: 5T088 ACR Abstract: RA–DX, Manifestations, & Outcomes IV: CV Co-Morbidities (2814–2819)
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Methotrexate (MTX) has been associated with reduced risk for cardiovascular disease (CVD) in several studies conducted among rheumatoid arthritis (RA) patients never exposed to biologic disease-modifying antirheumatic drugs (bDMARDS). Effect of concomitant MTX use on CVD risk among RA patients initiating bDMARDS remains unknown.
Methods: A retrospective cohort study was conducted using 2006-2015 Medicare claims data for RA patients. Follow up started at initiation (index date) and ended at earliest of 1) end of exposure of the specific bDMARDS agent (days of supply + 90 day extension), 2) switched to other bDMARDS or tofacitinib, 3) CVD event, 4) death date, 5) loss of Medicare coverage, 6) end of study (September 30, 2015). MTX use was defined as 1) concomitant MTX use, with prescription for MTX within 120 days after index date and 2) time varying MTX, defined as prescription date to prescription date plus days of supply without extension. For sensitivity analysis, a 90-day extension was added to days of supply. The primary outcome was composite of incident MI, incident stroke and fatal CVD. Fatal CVD were identified by a claims based algorithm with PPV ≥80%. Incidence rates (IR) and 95% confidence intervals (CI) were calculated using Poisson regression. Overall association between MTX use and risk of CVD were assessed using Cox regression. Given that the interactions between MTX and background bDMARDs was significant, we performed contrast (MTX Yes vs. No) to examine the association between MTX and risk for CVD for each underlying bDMARDS in one model. A subgroup analysis limited the cohort to RA patients with previous exposure to MTX was conducted to ensure consistency of findings.
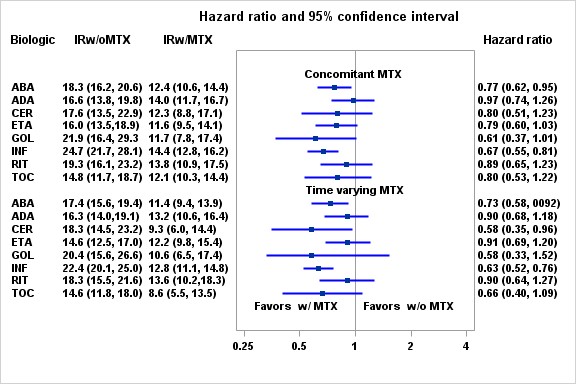
Results: A total of 88,255 DMARDS initiations (64,218 patients) were included in this study. The average age at initiation was 64.6 (12.3) years, 84.0% were female, 68.2% were non-Hispanic white. The crude IRs for CVD were 13.1 (95%CI: 12.2-14.0) and 18.7 (95%CI: 17.6-19.9) events per 1,000 person years (PY) for RA patients with and without concomitant MTX respectively. The crude IRs for CVD were 12.1 (95%CI: 11.1-13.2) and 17.9 (95%CI: 16.9-18.8) events per 1,000 PY for RA patients with and without time varying MTX respectively. IRs for individual bDMARDS are shown in figure. P-value for interaction between concomitant MTX and background bDMARDS was 0.0189 and p-value for interaction between time varying MTX and background bDMARDS was 0.0030. The contrast HRs for concomitant MTX ranged from 0.61 (0.37, 1.01) for golimumab initiators to 0.97 (0.74, 1.26) for adalimumab initiators (Figure). The contrast HRs for time varying MTX ranged from 0.58 (0.35, 0.96) for certolizumab initiators to 0.90 (0.68, 1.18) for adalimumab initiators. Results were robust in sensitivity and subgroup analyses.
Conclusion: Our observational study suggests an overall 23% reduction of CVD risk associated with concomitant MTX use. The effect sizes vary among background bDMARDS.
To cite this abstract in AMA style:
Xie F, Chen L, Levitan E, Muntner PM, Curtis JR. Methotrexate Use and the Risk for Cardiovascular Disease Among Rheumatoid Patients Initiating Biologic Disease-Modifying Anti-Rheumatic Drugs [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/methotrexate-use-and-the-risk-for-cardiovascular-disease-among-rheumatoid-patients-initiating-biologic-disease-modifying-anti-rheumatic-drugs/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/methotrexate-use-and-the-risk-for-cardiovascular-disease-among-rheumatoid-patients-initiating-biologic-disease-modifying-anti-rheumatic-drugs/