Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Liver test abnormalities are known to predict liver pathology in patients using methotrexate (MTX). Monitoring guidelines for MTX suggest frequent assessment of liver tests (AST and ALT). The frequency of liver test abnormalities and liver pathology (liver AEs) in patients using MTX varies substantially in prior studies and there are sparse placebo-controlled data on these issues. We examined liver AEs reported during follow-up in the Cardiovascular Inflammation Reduction Trial (CIRT), a randomized double-blind placebo-controlled trial of MTX for the prevention of cardiovascular disease.
Methods: The current analyses were pre-defined as secondary analyses of the CIRT trial (Ridker et al, NEJM 2019). AST and ALT were collected every 8-10 weeks during CIRT, dosages were adjusted by rheumatologists if liver tests were found to be abnormal, and work-up was recommended for patients with persistently elevated liver tests. Patients were randomized to receive MTX (target dose 15 to 20mg weekly) or placebo. Median trial follow-up was 2.3 years; maximum 5 years. All reports of liver AEs at routine visits or as hospitalized serious AEs were followed up with a request for medical records. These events were adjudicated using a standardized assessment by an internist (DHS) and liver specialist (AR). We examined the frequency in all patients of AST and ALT abnormalities ( >upper limits of normal, ULN) and liver pathology, defined as cirrhosis, non-alcoholic steatohepatitis (NASH), and other. AST and ALT abnormalities were also categorized as mild ( >1- 2 x ULN), moderate ( >2-3 x ULN), or severe ( >3 x ULN). The relative rates of these outcomes were estimated using hazard ratios (HR) in adjusted Cox regression. Adjusted Cox regression was also used to examine risk factors for first liver test abnormalities among patients in the MTX arm.
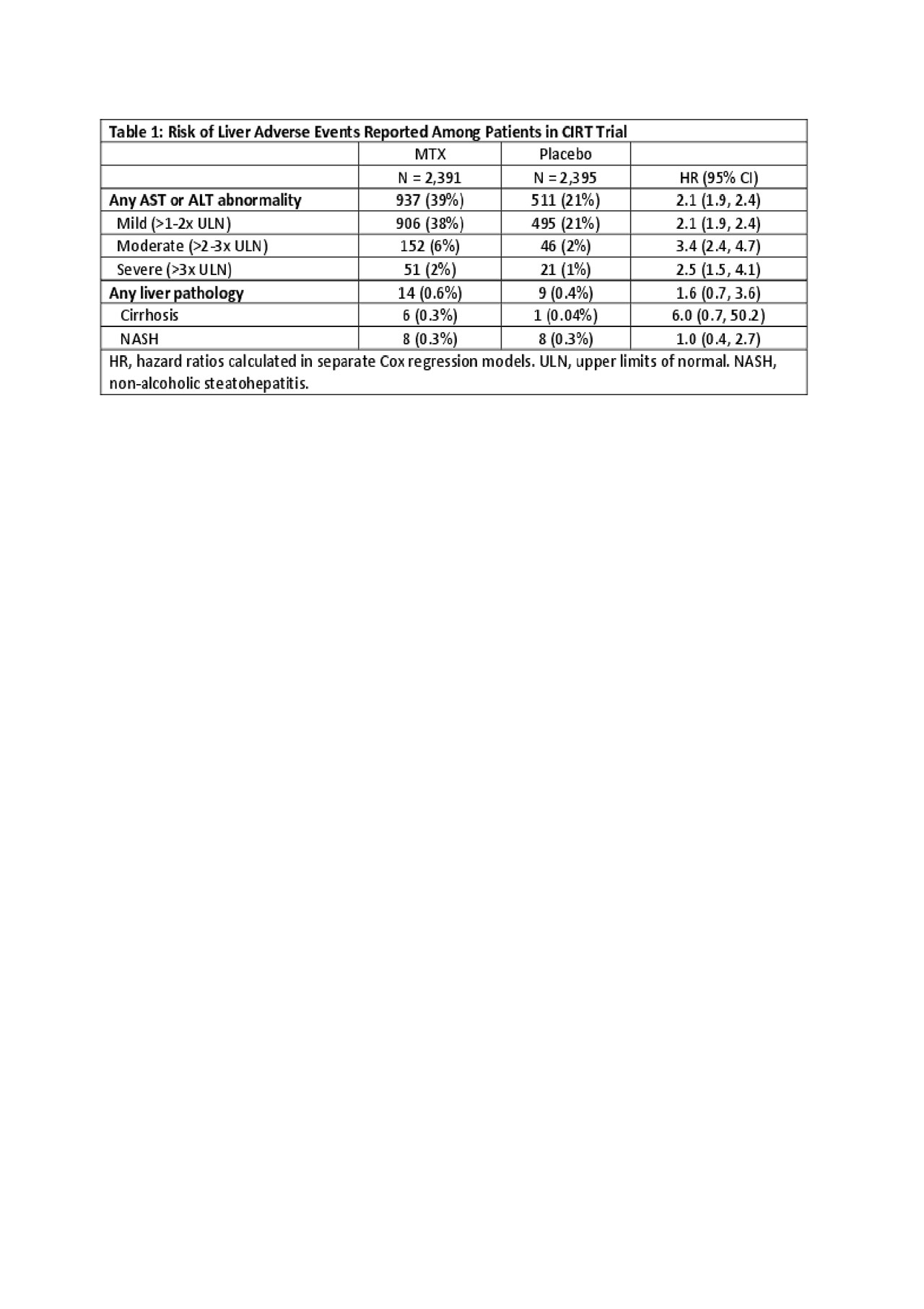
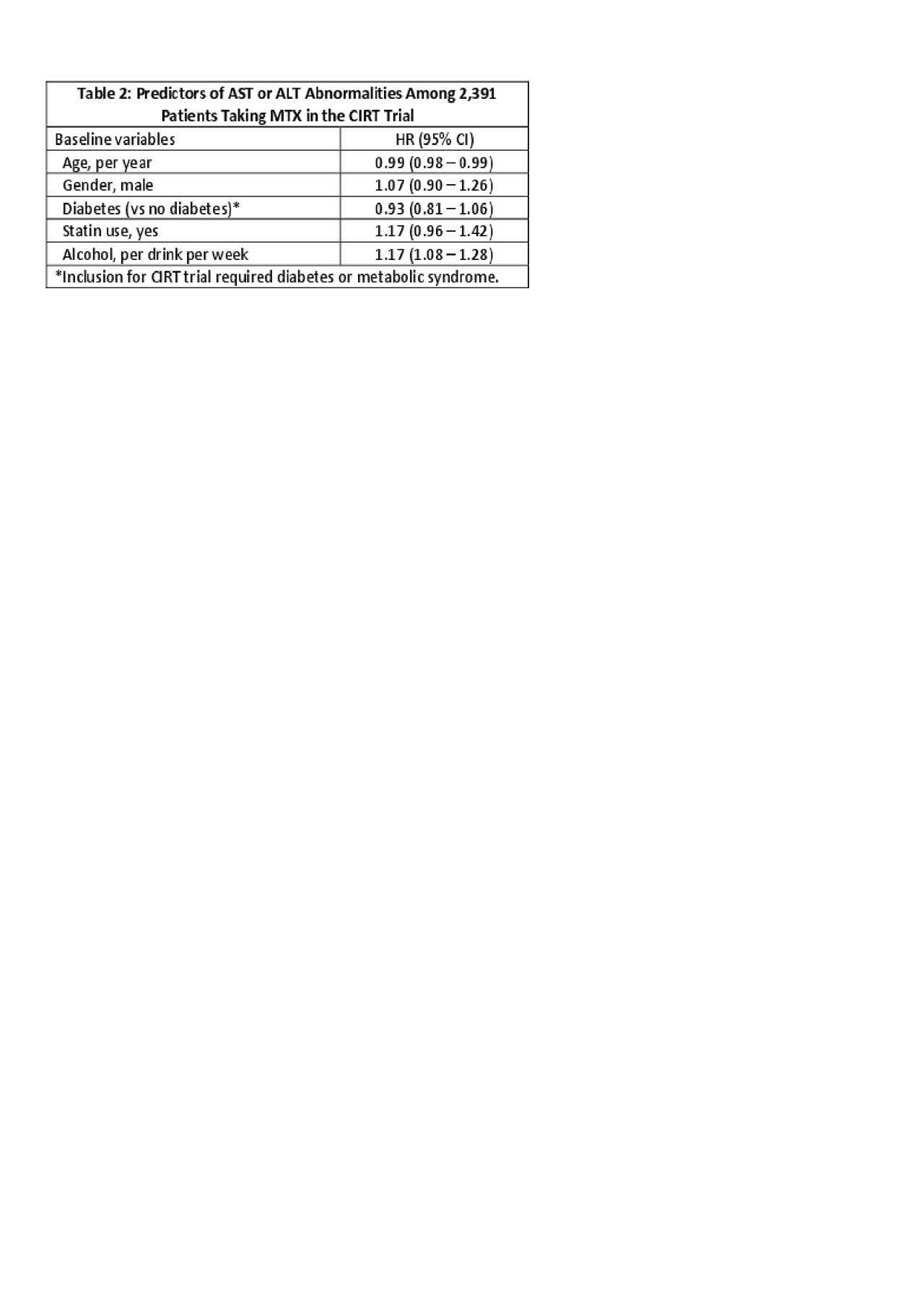
Results: 2,391 patients were randomized to MTX (median dosage of 16mg weekly) and 2,395 to placebo. During follow-up, in the MTX arm, 937 (39%) experienced an AST or ALT abnormality with a rate of abnormal tests (first or recurrent) of 11.5 per 100 tests (95% CI 11.1-11.9). In the placebo arm, 511 (21%) experienced an abnormal test with a rate (first or recurrent abnormality) of 5.1 per 100 tests (95% CI 4.8-5.3). The hazard ratio (HR) for first abnormal liver tests was 2.1 (95% CI 1.9-2.4) in MTX versus placebo. The risk for abnormal liver tests of different severity are shown in Table 1. There were 14 (0.6%) patients in the MTX arm and 9 (0.4%) in the placebo arm that were diagnosed with liver pathology during follow-up; the HR for these events was 1.6 (95% CI 0.7-3.6). These events were categorized as cirrhosis (n=6, 0.3% in MTX vs n=1, 0.04% in placebo) and NASH (n=8, 0.3% in MTX vs n = 8, 0.3% in placebo) (see Table 1). Predictors of ALT or AST abnormalities in the MTX arm included younger age and more alcohol intake at baseline (see Table 2).
Conclusion: Compared to placebo, the risk of abnormal AST or ALT was increased two to three-fold with MTX use; most abnormal values were only mildly elevated. More alcohol intake and younger age were the only clear risk factors for abnormal liver tests among patients randomized to MTX. We found few cases of cirrhosis during follow-up in either arm, but more in the MTX arm.
To cite this abstract in AMA style:
Solomon D, Paynter N, Rutherford A, Glynn R, MacFadyen J, Lu F, Xu C, Karlson E, Ridker P. Methotrexate Liver Toxicity in a Large Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/methotrexate-liver-toxicity-in-a-large-randomized-controlled-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/methotrexate-liver-toxicity-in-a-large-randomized-controlled-trial/