Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Large interpatient variability in Methotrexate (MTX) absorption and intracellular activation to methotrexate polyglutamates (MTXPGs) can explain, in part, the large spectrum of MTX response in patients with rheumatic diseases. We sought to identify the contributors to the large inter-subject variability in MTX exposure during the course of clinical care of patients with rheumatoid diseases.
Methods: Red blood cells (RBC) MTXPG levels were requested by treating physicians during the course of clinical care of patients with rheumatic diseases. Of all RBC MTXPG measurements performed, a total 15,428 measurements from 12,725 patients were available with weekly MTX dosing information as provided by the requesting physicians. In a subset of 2571 test results from 2261 patients the route of MTX administration (oral vs. parenteral) was provided. MTX RBC MTXPG3 levels were measured in our accredited clinical laboratory using liquid chromatography and reported as nmol/L packed RBCs. A dose normalized polyglutamation rate defined as the amount of MTXPG produced per mg MTX administered (nmol/Lxmg) was calculated. To maintain patient privacy, all specimens were de-identified prior to the analysis. The statistical analyses consisted of multivariate linear regressions, and non parametric tests to assess differences between groups.
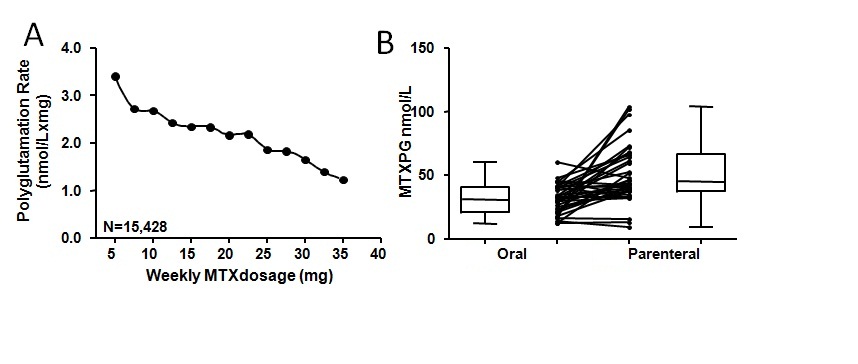
Results: There was a large interpatient variability in MTX polyglutamation. Median MTX dose administered was 17.5 mg/week (Interquartile [IQ] range: 15-20 nmol/L), median MTXPG levels were 36 nmol/L (IQ range: 21-54 nmol/L) and median MTX polyglutamation rate was 2.1 nmol/Lxmg (IQ: 1.2-3.0 nmol/Lxmg). The proportion of patients having undetectable MTXPG (<2 nmol/L), a potential indicator of non-adherence was 7.6%. Multivariate linear regression analysis revealed that lower MTX polyglutamation rates was associated with higher MTX dosage (p<0.001), lower patient age (p<0.001) and oral (vs. parenteral) administration of MTX (p<0.01) (Global R2=0.12; n=2571). The impact of MTX dosing on MTX polyglutamation is highlighted in the Figure (panel A). In 31 patients who switched from oral to parenteral MTX without change in MTX dose (median 20 mg/week, range 15-25 mg), RBC MTXPG levels were determined 18 weeks (median) apart (range 8-65 weeks). The switch from oral to parenteral MTX was associated with a 1.4-fold increase in MTXPG levels (32 nmol/L [range 12-60 nmol/L] vs. 44 nmol/L [range 9-102 nmol/L]; p<0.01) (Figure, panel B).
Conclusion: These findings are consistent with the notion that MTX polyglutamation is a saturable process at higher MTX dosage. Switching from oral to parenteral administration of MTX produces higher levels of RBC MTX polyglutamates.
Disclosure:
R. A. Kaplan,
None;
B. Freyne,
None;
D. Barken,
Exagen,
3;
T. Dervieux,
Exagen,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/methotrexate-exposure-assessment-in-routine-clinical-rheumatology-practice-effect-of-dosing-and-route-of-administration-on-polyglutamate-levels/