Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Methotrexate is commonly prescribed for a variety of diseases including rheumatoid arthritis. Methotrexate has frequently been implicated as a causative agent in interstitial lung disease. Patients with rheumatoid arthritis can develop pulmonary disease for a variety of reasons including infection and rheumatoid interstitial lung disease. Distinguishing methotrexate lung toxicity from other aetiologies is vital in the clinical setting as methotrexate is an effective treatment for rheumatoid arthritis. The aim of this study was to evaluate if methotrexate is associated with an increased risk of lung disease in rheumatoid arthritis.
Methods:
We performed a systematic literature search from 1st January 1990 to 31st March 2011 using Pubmed and Cochrane databases. The inclusion criteria for study selection were (1) randomised controlled trials; (2) human patients with rheumatoid arthritis; (3) studies in English; (4) studies consisting of a minimum of two arms, at least one receiving methotrexate and at least one not receiving methotrexate; (5) studies including only adults(>18 years); (6) trials of ³6 months duration; (7) studies of ³100 patients; (8) studies reporting respiratory side effects for methotrexate and comparator groups individually. Random effects meta-analysis using the Mantel-Haenszel method was used to assess total respiratory events, infectious respiratory events and non-infectious respiratory events. Results were expressed as relative risks (RR) with 95% confidence intervals.
Results:
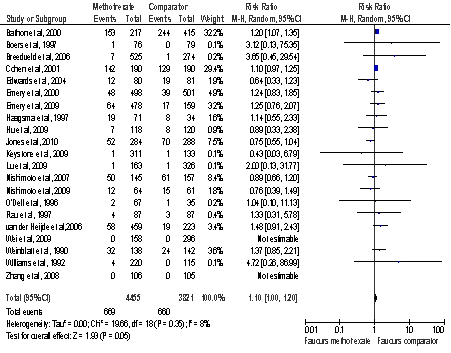
21 studies with a total of 8276 participants met our study’s inclusion criteria and were included in the meta-analysis. Methotrexate was not associated with an increased risk of total adverse respiratory events, RR 1.1 (95% CI 1.0-1.2, I2=8%), Figure 1. No difference was identified in the risk of adverse events when analysed separately for infectious and non-infectious outcomes, RR 1.09 (95% CI 1.0-1.19, I2=0%) and 1.11 (95% CI 0.68-1.81, I2=48%) respectively. There was no difference in the risk of pulmonary death between the 2 groups, RR 1.41 (95% CI 0.43-4.63, I2=0%). A subgroup analysis of studies specifically reporting pneumonitis revealed an increased risk in the methotrexate group, RR 6.99 (95% CI 1.57-31.05, I2=0%); however none of the publications since 2001 in our study reported any cases of pneumonitis.
Conclusion:
Our study did not find a significant increase in the risk of lung disease in RA patients treated with methotrexate. One subgroup analysis showed a significant increased risk of pneumonitis; however publications after 2001 have not reported this association.
Figure 1: Total Respiratory Events
Disclosure:
R. Conway,
Roche Pharmaceuticals,
2,
UCB Pharma,
2,
Merck Pharmaceuticals,
7;
C. Low,
Roche Pharmaceuticals,
2,
UCB Pharma,
2,
Merck Pharmaceuticals,
7;
R. J. Coughlan,
None;
M. O’Donnell,
None;
J. J. Carey,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/methotrexate-and-interstitial-lung-disease-in-rheumatoid-arthritis-a-systematic-literature-review-and-meta-analysis/