Session Information
Date: Monday, November 13, 2023
Title: (1554–1578) Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Behçet’s disease (BD) is characterized by great clinical heterogeneity. The therapeutic strategy for BD could be tailored to the specific needs of the patient, based on clinical phenotypes, as we often find refractory cases with overlapping of different clinical manifestations. Eular recommendations on the management of BD were updated in 2018 and did not include methotrexate (MTX) as a therapeutic strategy, despite being a widely used drug in Rheumatology. OBJECTIVES: To describe the efficacy and safety of MTX treatment in patients diagnosed with BD.
Methods: We performed a multicentre retrospective observational study that included 68 BD patients (ICBD 2013) on MTX treatment. The indication of MTX was based on the criteria of the treating physician with the informed consent of the patient. Statistical analysis was performed by T-test and Fisher’s exact test to compare quantitative and qualitative variables. Values of p < 0.05 were considered statistically significant.
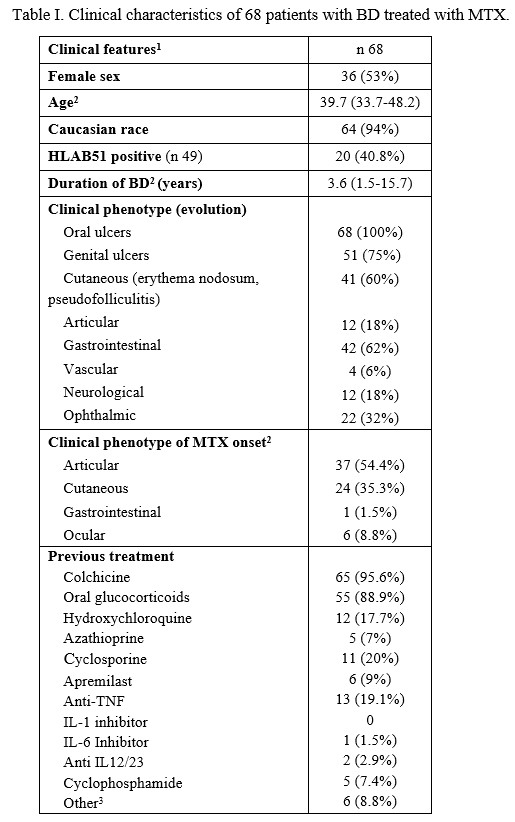
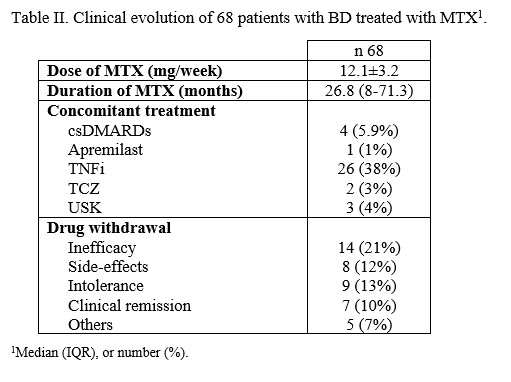
Results: 68 patients diagnosed with BD and treated with MTX was evaluated, 53% women, 94% caucasians, with a median age of 39.7 (33.7-42.8) years and 3.6 (1.5-15.7) years of disease progression at MTX onset. Table I summarises the main general and clinical features at baseline. Previously to MTX, patients had received colchicine (95.6%), oral glucocorticoids (80.9%, maximum median dose of 20 [10-30] mg/day), hydroxychloroquine (17.7%) and csDMARDs (35.8%). In addition, 6 patients had received apremilast and 13 had received bDMARDs. MTX was started due to joint phenotype (54.4%), refractory oral and/or genital aphthosis (35.3%) and ocular involvement (8.8%). The mean dose of MTX was 12.6±3.6 mg/week. Survival at 3, 6 and 12 months was 94% (61/68), 84% (57/68) and 68% (42/68). The median duration of MTX was 26.8 (8-71.3) months, and treatment was discontinued in 43 patients (63.2%) (Table II).
To assess efficacy, patients who presented early intolerance or adverse effects (< 6 months) were eliminated (7/68).46% of patients (28/61) received MTX for joint involvement and 54% of patients (33/61) for other reasons. Those who received treatment for arthritis had a higher response rate (24/28) compared to those who received MTX for other clinical manifestations (19/33) (p=0.016). There were no significant differences between these two groups in terms of maximum dose of methotrexate or the use of concomitant biologic therapy.
Conclusion: Despite not being included as a therapeutic strategy in the latest Eular 2018 recommendations, methotrexate appears to be an effective treatment in patients with BD, especially against the articular phenotype, with a good safety profile.
To cite this abstract in AMA style:
Retuerto-Guerrero M, Díez Álvarez E, Narvaez J, Fornons Servent R, Moreno Vilchez C, Graña Gil J, Couto Lareo U, San José-Méndez C, Moriano Morales C. Methotrexate: A Safe and Effective Therapeutic Alternative in Behçet´s Disease [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/methotrexate-a-safe-and-effective-therapeutic-alternative-in-behcets-disease/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/methotrexate-a-safe-and-effective-therapeutic-alternative-in-behcets-disease/