Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Patients with rheumatoid arthritis (RA) experience adverse events (AEs) attributed to both the disease and its treatment. Tofacitinib is a novel oral Janus kinase inhibitor being investigated as a targeted immunomodulator and disease-modifying therapy for RA. To contextualize events within the tofacitinib clinical trial program, a meta-analysis was completed in patients with RA receiving biologic drug therapy within a randomized clinical trial (RCT) setting to identify and quantify safety endpoints of: malignancies excluding non-melanoma skin cancer (NMSC), serious infections (SIs), and serious AEs (SAEs).
Methods: Medline, Embase, PubMed, and summary basis of approvals from regulatory submissions were searched to identify RCTs for abatacept, rituximab, etanercept, infliximab, certolizumab, golimumab, adalimumab, and tocilizumab in RA. The search identified >300 papers from which data from 80 RCTs, representing more than 31,000 subjects, were extracted for analysis for the three endpoints. Non-RCTs, long-term extensions, and observational studies were excluded from the literature results. Tofacitinib results from five RCTs (Phase 3 [P3]) are presented. The dependent variable for the analysis was the incidence rate (IR) of an event/100 patient-years (pt-yrs). Data were analyzed using a random effects meta‑analysis model. The IR data were log transformed to avoid negative confidence intervals (CIs). An imputation methodology was applied to account for IRs of zero and a sensitivity analysis was performed to assess the effects of adjustment on the individual and overall mean, as well as the impact on the estimated variance surrounding each study arm.
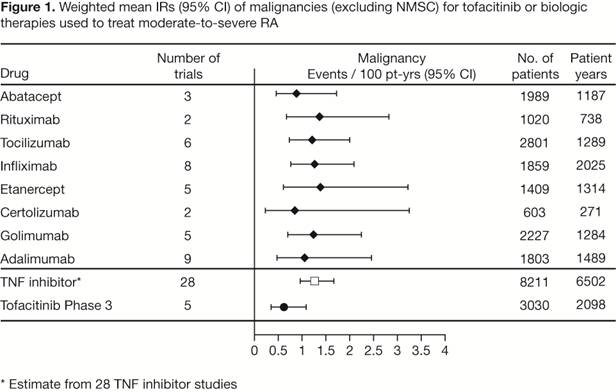
Results: Estimated IRs for endpoints of malignancies, SIs, and SAEs revealed similar rates among biologic therapies used to treat RA. Across all biologic therapies, point estimates ranged from 0.8 to 1.4 events/100 pt-yrs for malignancies; 2.5 to 6.5 for SIs; and 10.7 to 22.0 for SAEs. Event rates for tofacitinib were 0.62 (95% CI 0.36, 1.07) events/100 pt-yrs for malignancies (Figure 1); 2.91 (2.27, 3.74) events/100 pt-yrs for SIs; and 10.3 (9.00, 11.78) events/100 pt-yrs for SAEs, all in P3. The 95% CIs for tofacitinib were contained within the range of published estimates.
Conclusion: This RCT meta-analysis provides a quantitative assessment of the incidence of important safety events reported with therapies for the treatment of RA. Overall, tofacitinib event rates for malignancies, SIs, and SAEs were comparable to published rates for approved biologic therapies. Future analyses are warranted to estimate relative effects (treatment comparisons), model studies with no events (IR=0), and account for study population differences.
Disclosure:
S. Ahadieh,
Pfizer, Inc.,
1,
Pfizer, Inc.,
3;
T. Checchio,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
T. Tensfeldt,
Pfizer, Inc.,
1,
Pfizer, Inc.,
3;
J. French,
Pfizer, Inc.,
1,
Pfizer, Inc.,
3,
Pfizer, Inc.,
5;
S. Krishnaswami,
Pfizer, Inc.,
1,
Pfizer, Inc.,
3;
R. Riese,
Pfizer, Inc.,
1,
Pfizer, Inc.,
3;
S. Menon,
Pfizer, Inc.,
1,
Pfizer, Inc.,
3;
M. G. Boy,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
J. L. Geier,
Pfizer, Inc.,
1,
Pfizer, Inc.,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/meta-analysis-of-malignancies-serious-infections-and-serious-adverse-events-with-tofacitinib-or-biologic-treatment-in-rheumatoid-arthritis-clinical-trials/