Session Information
Date: Tuesday, October 23, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Pain is a core domain of psoriatic arthritis (PsA).1 Rapid, sustained pain reduction is a priority for patients (pts) and physicians when choosing treatment. Tofacitinib is an oral Janus kinase inhibitor for the treatment of PsA. This post hoc analysis aimed to estimate time to clinically meaningful pain improvement with tofacitinib.
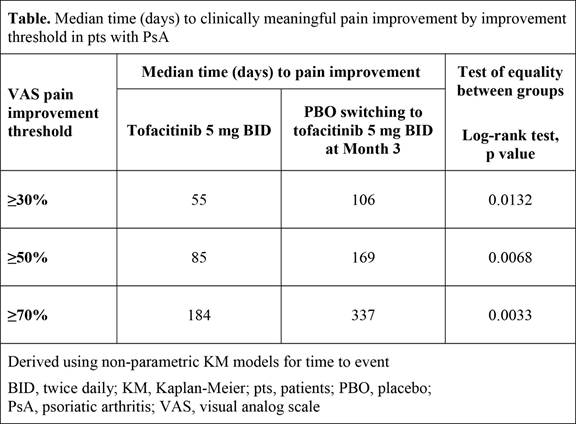
Methods: Data were analyzed from 2 Phase 3 studies of tofacitinib in pts with active PsA and an inadequate response to ≥1 conventional synthetic DMARD (OPAL Broaden; NCT01877668; 12-month study) or to ≥1 tumor necrosis factor inhibitor (OPAL Beyond; NCT01882439; 6-month study). Pts treated with tofacitinib 5 mg twice daily (BID) and placebo (PBO) advanced to tofacitinib 5 mg BID at Month 3 (PBO-tofacitinib), in combination with csDMARDs, were included in this analysis. Current arthritis pain severity was reported by pts using a 100 mm visual analog scale, where higher scores indicated greater severity of pain. Pain improvement was defined as the first post-baseline improvement of ≥30% (meaningful change), ≥50% (substantial change), or ≥70% relative to baseline.2 Time-to-event analyses were performed using a Kaplan-Meier (KM) method on pooled data. Descriptive analyses of the rate of improvements by study were performed.
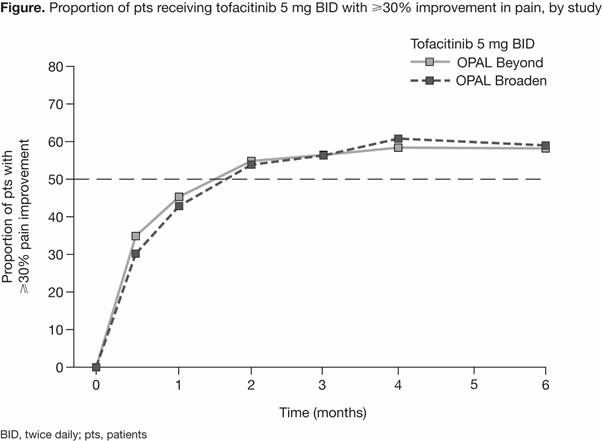
Results: Overall, 354 pts were available for analysis. Rates of pain improvement over time with tofacitinib 5 mg BID were approximately the same in both studies (Figure). By Month 1, ≥40% of pts experienced ≥30% pain improvement, and by Month 2, approximately 55% of pts experienced ≥30% pain improvement (Figure). KM analyses showed that pts receiving tofacitinib 5 mg BID achieved improvements in pain severity of 30–70% significantly faster compared with pts in the PBO-tofacitinib group (Table). The median time to ≥30% pain improvement was 55 days in the tofacitinib 5 mg BID group and 106 days in the PBO-tofacitinib group (pts switched to tofacitinib 5 mg BID at Month 3; p=0.0132). Similar trends between treatment groups were observed across other pain improvement thresholds.
Conclusion: In pts with active PsA, faster, clinically meaningful pain improvements were reported in pts receiving tofacitinib 5 mg BID vs pts receiving PBO who switched to tofacitinib 5 mg BID at Month 3. After switch from PBO to active treatment, pain improvement was observed in line with pts receiving active treatment from Day 0. To achieve pain improvement at greater thresholds, longer duration of active treatment was required.
1. Orbai AM et al. Ann Rheum Dis 2017; 76: 673-80.
2. Dworkin RH et al. J Pain 2008; 9: 105-21.
To cite this abstract in AMA style:
de Vlam K, Ogdie A, Bushmakin AG, Cappelleri JC, Fleischmann R, Taylor PC, Azevedo VF, Fallon L, Maniccia A, Woolcott J, Mease PJ. Median Time to Pain Improvement in Patients with Psoriatic Arthritis Treated with Tofacitinib [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/median-time-to-pain-improvement-in-patients-with-psoriatic-arthritis-treated-with-tofacitinib/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/median-time-to-pain-improvement-in-patients-with-psoriatic-arthritis-treated-with-tofacitinib/