Session Information
Date: Sunday, November 10, 2019
Title: RA – Diagnosis, Manifestations, & Outcomes Poster I: Risk Factors, Predictors, & Prognosis
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Up to 80% of rheumatoid arthritis patients report clinically relevant fatigue. Fatigue is a complex multi-factorial process that can result in adverse affects on patients’ physical and emotional well-being. The purpose of this study was to examine the relationship between disease activity and fatigue over time in early rheumatoid arthritis (ERA).
Methods: Data were from patients with ERA (symptoms ≤ 12 months) enrolled in the Canadian Early Arthritis Cohort (CATCH). CATCH participants completed repeat clinical assessments, laboratory investigations and self-reported questionnaires including rating their fatigue over the past week using a 0-10 point numerical rating scale (NRS). Fatigue severity was classified as low (≤2); moderate ( >2 but < 5) and high (≥5) based on other published RA studies. T-tests and repeated measures ANOVA were used to compare differences in fatigue in patient who did vs. did not achieve a low disease state using ESR (DAS28 ≤ 3.2) or REM (DAS28 < 2.6) within 3-months of cohort entry. Paired t-tests were used to compare fatigue at different time points in patients achieving remission (DAS < 2.6) at three or more consecutive visits within the first year of follow-up.
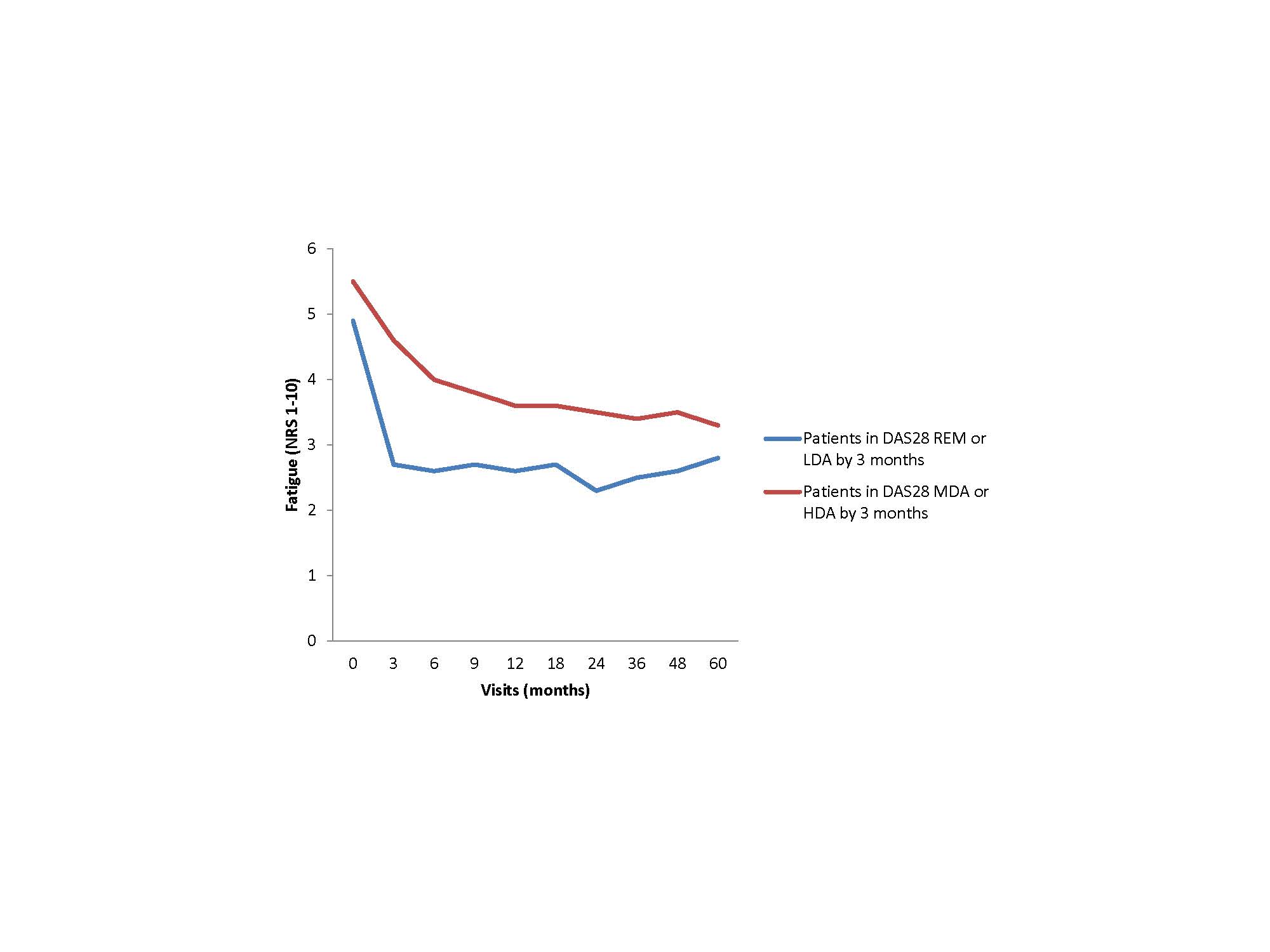
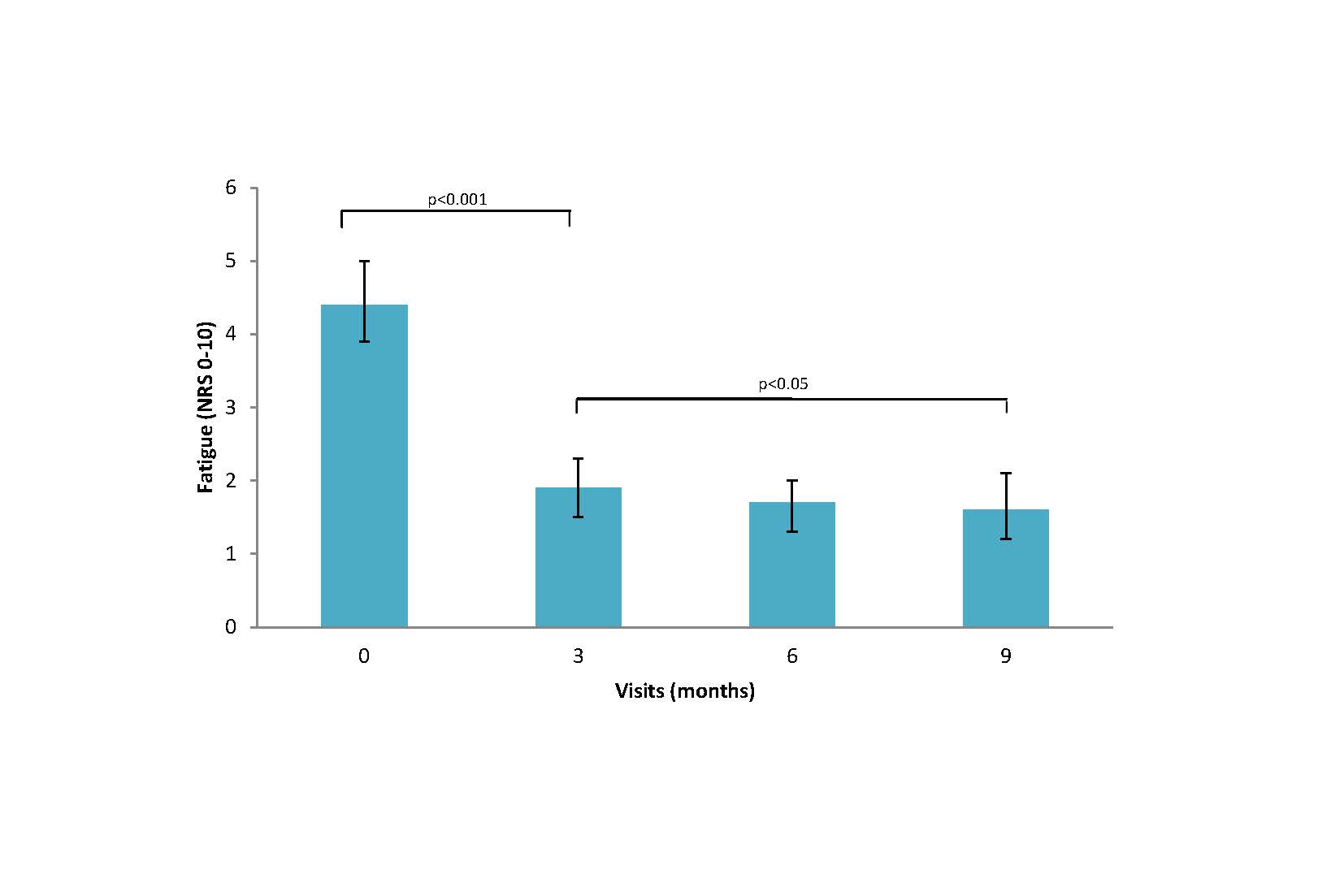
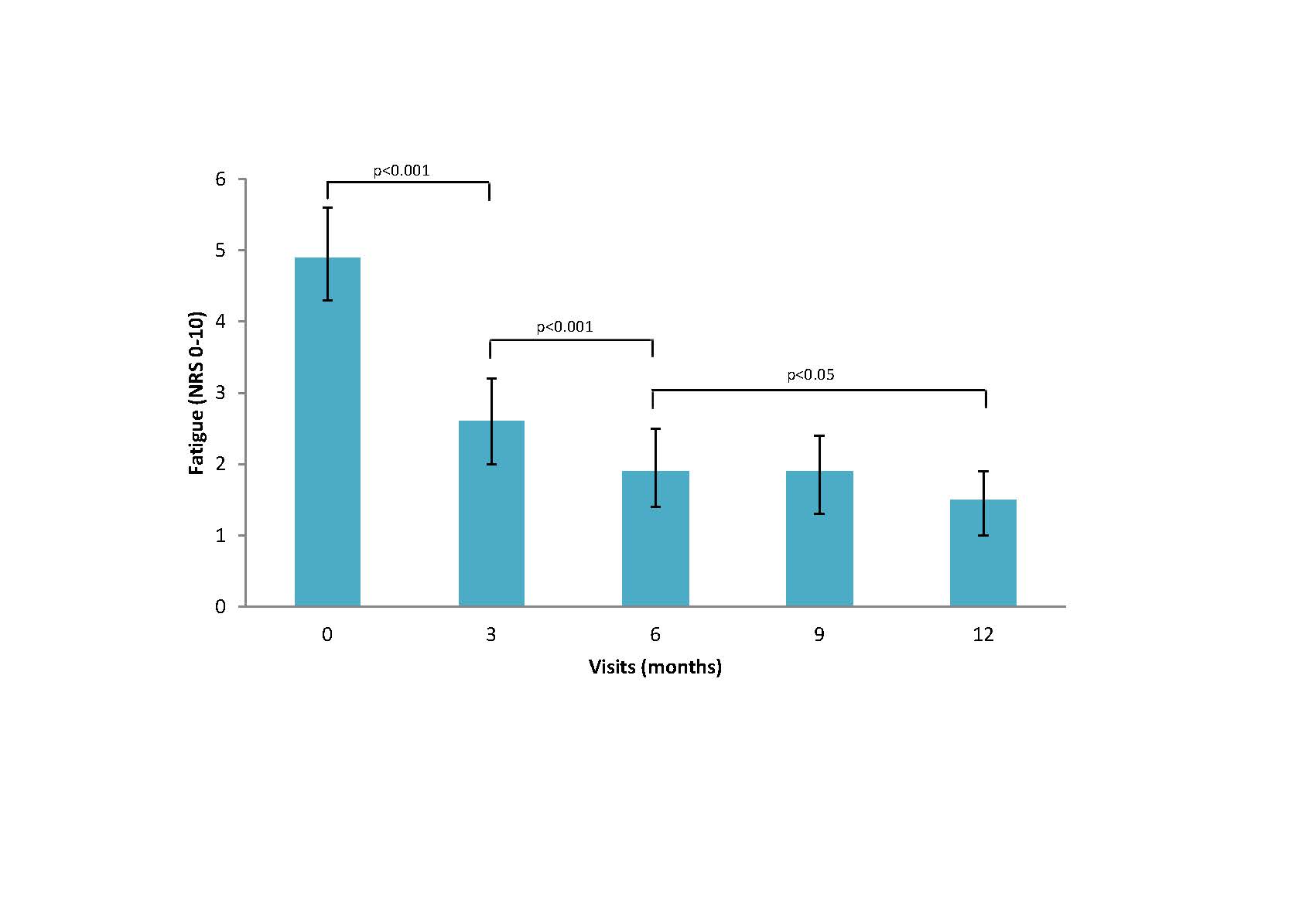
Results: Of the 1864 patients included, 1640 (88%) met ACR criteria for RA, 1342 (72%) were women and most had moderate-high baseline disease activity with a mean (SD) DAS28 of 4.9 (1.5). Fatigue was common with 19% reporting moderate and 59% high fatigue at baseline. Patients who reported low fatigue severity by three months continued to have significantly lower fatigue throughout follow-up compared to those with moderate or high fatigue (p< 0.001). Patients who achieved DAS28 REM or LDA within 3-months of cohort entry (N=539) had significantly lower mean fatigue throughout 5 years of follow-up compared to those who did not achieve REM or LDA within 3 months (p< 0.001) (Figure 1). Patients who achieved sustained remission in the first 3-months of cohort entry (N=236) had significantly decreased fatigue at time of first achieving DAS REM and 6 months after initially achieving DAS REM (Figure 2). Patients who first achieved sustained remission after 6-months of cohort entry (N= 141) had significantly decreased fatigue at both 3 and 6-months after cohort entry as well as 6-months after the first time in DAS REM (Figure 3).
Conclusion: Fatigue is common in ERA and is significantly decreased at time of first remission in those with sustained remission within the first year of diagnosis. Maximal improvement in fatigue lags behind with further improvement in fatigue seen 6 months after first remission. Early treatment response within 3-months was associated with short and long-term improvements in fatigue over time. This may have implications for counselling patients.
DAS28-ESR-: disease activity score in 28 joints using Erythrocyte Sedimentation Rate; REM: remission; LDA: low disease activity; MDA: moderate disease activity; HDA: high disease activity.
To cite this abstract in AMA style:
Holdren M, Schieir O, Bartlett S, Bessette L, Boire G, Hazlewood G, Hitchon C, Keystone E, Tin D, Thorne C, Bykerk V, Pope J, (CATCH) Investigators C. Maximal Improvement in Fatigue Lags Behind Achievement of Sustained Remission in Early Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/maximal-improvement-in-fatigue-lags-behind-achievement-of-sustained-remission-in-early-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/maximal-improvement-in-fatigue-lags-behind-achievement-of-sustained-remission-in-early-rheumatoid-arthritis/