Session Information
Date: Monday, November 14, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Inhibition of GM–CSFR-α is a novel approach to treat RA. Mavrilimumab, an investigational human monoclonal antibody targeting GM–CSFR-α, has demonstrated efficacy and an acceptable safety profile in prior 12- and 24‑week studies. Here, the long-term (LT) efficacy and safety of mavrilimumab 100 mg every other week (eow) is evaluated for up to 158 (median = 122) weeks treatment in patients (pts) with moderate to severe RA.
Methods: Adult pts were enrolled into this open-label extension (OLE) study (NCT01712399) after completing either EARTH EXPLORER 1 or 2 (NCT01706926; NCT01715896), or transferred from Week 12 onwards because of inadequate response. Pts received subcutaneous mavrilimumab 100 mg eow, consistent with the highest dosage in the Phase IIa study. The LT risk:benefit ratio of mavrilimumab was assessed via evaluation of treatment-emergent adverse events (TEAEs), serious TEAEs (TESAEs), and pulmonary events. Exploratory LT efficacy was measured using clinical (DAS28–CRP and ACR responses) and patient-reported outcomes [PROs: HAQ-DI; Short Form Health Survey (SF)-36; Functional Assessment of Chronic Illness Therapy (FACIT) fatigue]; efficacy duration was assessed via evaluation of major clinical response (maintenance of ACR70 for 24 weeks) and maintenance of DAS28–CRP <2.6. Radiographic progression (modified total Sharp score [mTSS]) was assessed in pts initially enrolled in EARTH EXPLORER 1.
Results: 397 pts were included in the OLE study. Between the Phase IIb and OLE studies, 442 pts received mavrilimumab, with a cumulative safety exposure of approximately 900 pt-years (yrs). Across all time points, the most frequently reported TEAEs for all pts (n [rate/100 pt-yrs]) were nasopharyngitis (69 [7.68]), bronchitis (51 [5.68]), RA (44 [4.90]), urinary tract infection (38 [4.23]), and hypertension (38 [4.23]); the serious infection rate was 1.56/100 pt-yrs. Treatment with mavrilimumab was not associated with substantial effects on pulmonary safety, including function; monocytopenia was not reported and shifts in laboratory values were not clinically significant. At Week 122, ACR20/50/70 responses were achieved in approximately 80%, 50%, and 30% of pts, respectively; 65% of pts achieved DAS28−CRP <3.2 and 41% of pts achieved DAS28−CRP <2.6. In addition, efficacy was demonstrated across PRO endpoints; mean FACIT fatigue and SF-36 components showed improvements from baseline, which were maintained through 122 weeks (Table).
Conclusion: Mavrilimumab demonstrated sustained efficacy and an acceptable safety profile for up to 158 (median = 122) weeks in pts with moderate to severe RA. Mavrilimumab 100 mg eow is suboptimal compared with 150 mg eow in DMARD-IR pts; however, efficacy results remain comparable with previous studies, validating the use of mavrilimumab to target GM–CSFR-α. ^Joint senior authors. 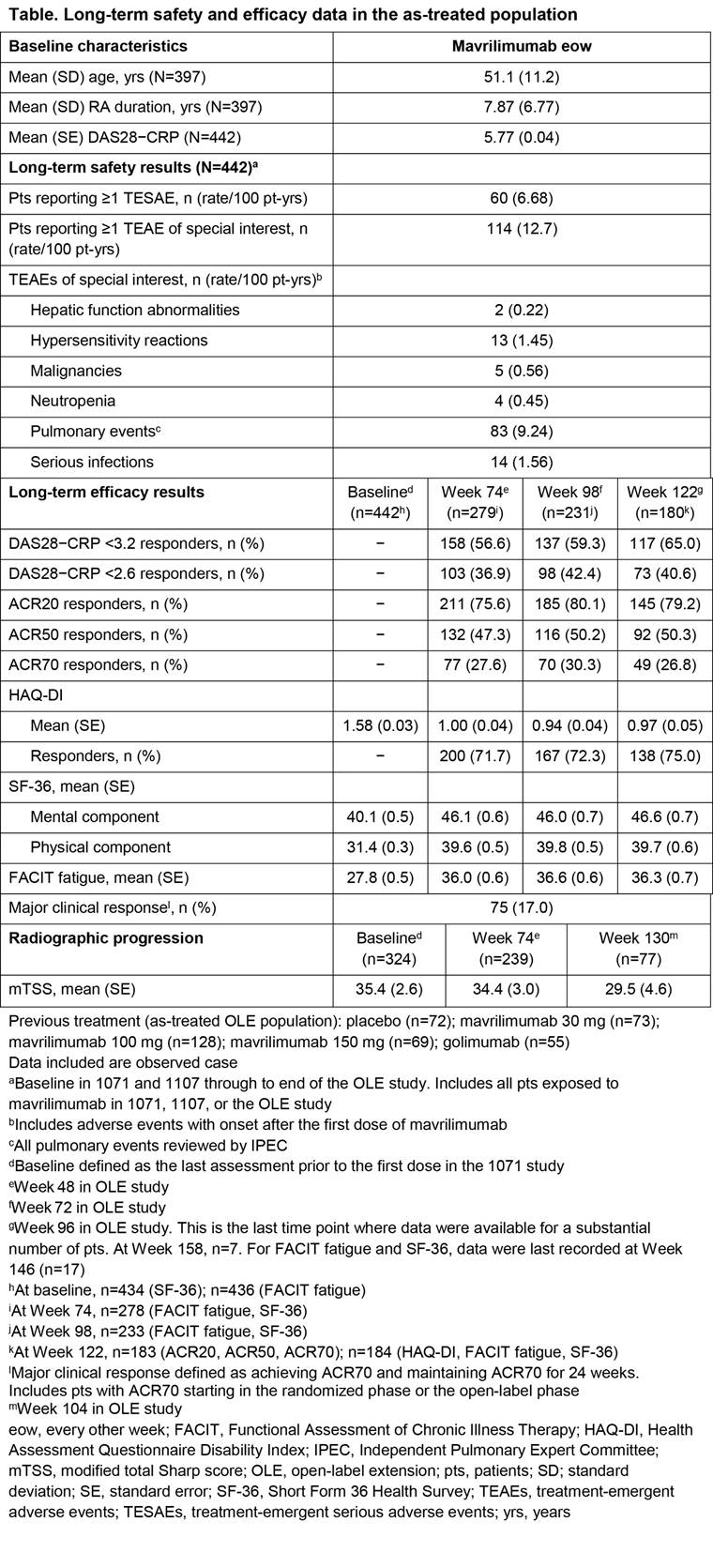
To cite this abstract in AMA style:
Burmester G, McInnes I, Kremer J, Miranda P, Vencovský J, Godwood A, Albulescu M, Close^ D, Weinblatt M. Mavrilimumab, a Fully Human Granulocyte-Macrophage Colony-Stimulating Factor Receptor-α Monoclonal Antibody: Long-Term Safety and Efficacy for up to 158 Weeks of Treatment in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/mavrilimumab-a-fully-human-granulocyte-macrophage-colony-stimulating-factor-receptor-%ce%b1-monoclonal-antibody-long-term-safety-and-efficacy-for-up-to-158-weeks-of-treatment-in-patients-with-rheuma/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mavrilimumab-a-fully-human-granulocyte-macrophage-colony-stimulating-factor-receptor-%ce%b1-monoclonal-antibody-long-term-safety-and-efficacy-for-up-to-158-weeks-of-treatment-in-patients-with-rheuma/
