Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Reports of positive effects of allogenic mesenchymal stem cells (MSCs) in treating refractory lupus, from a single center in China, led us to investigate the clinical and immune effect of a single infusion of 1×106 MSCs per kg umbilical cord MSCs in a Phase I trial of 6 patients with refractory lupus. We report the immune effects of MSC infusions on 6 patients; 5 met the primary outcome of a Systemic Lupus Response Index-4 (i.e. SRI-4). Clinical responses are submitted separately.
Methods: We assessed B and T cell subsets at Wks 0, 4, 8 and 24 by Flow. We measured levels of TGFb and glycoprotein A repetitions predominant (GARP) in the serum by ELISA as the hypothesized immune effector mechanism of MSCs. B cell assays were performed at Emory; 3 Wk. 8 samples were undeliverable due to winter storms.
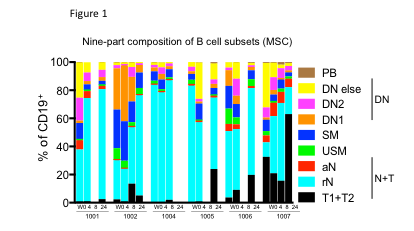
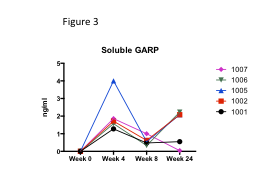
Results: B cell subsets were notably affected by MSC infusion with a significant decrease in CD27 IgD double negative B cells (DN) with a concomitant increase in resting naïve B cells (rN) in 4/6 (Fig. 1). Of particular note are the diminishing subsets with an activated phenotype (CD11c+CD21-), including DN2 and activated naïve (aN) B cells. Patient 004 was the single treatment failure and her B cell subsets, with a high percentage of rN B cells and few DN/aN B cells at baseline, did not change. We assessed for changes in Tregs, Th1, Th2, TFH and Th17 subsets. 2/6 patients had increases in Tregs (i.e. patients 001, Fig. 2) and helios+ T regs (Fig. 2), but there were no major shifts in subsets as seen in B cells. GARP is highly expressed on MSCs and a major regulator of TGFb bioactivity. GARP impacts Treg development, B cell activation and development of autoimmunity. We assessed if MSC infusions impacted serum GARP or TGFb levels. There were significant increases in serum GARP (Fig. 3, p<0.01) and TGFb levels (not shown) following MSCs.
Conclusion: MSC infusions in a Phase I open label trial in refractory lupus led to clinical improvement in 5/6 patients and significant changes in immune cell subsets with a shift from activated B cell subsets to resting naïve B cells. Serum GARP and TGFb levels increased post MSC infusion serving perhaps as the proximate immune effector mediators. A Phase II double blind placebo controlled multi-center trial of UC-MSCs in 81 refractory lupus patients will be starting this summer.
To cite this abstract in AMA style:
Wallace Fugle C, Wei C, Sanz I, Li Z, Paulos C, Wyatt M, Kamen DL, Gilkeson GS. Marked Immune Cell Subset Changes in Refractory Lupus Patients in a Phase I Trial of Allogenic Mesenchymal Stem Cells [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/marked-immune-cell-subset-changes-in-refractory-lupus-patients-in-a-phase-i-trial-of-allogenic-mesenchymal-stem-cells/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/marked-immune-cell-subset-changes-in-refractory-lupus-patients-in-a-phase-i-trial-of-allogenic-mesenchymal-stem-cells/