Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: PsA is a complex inflammatory condition affecting both the skin and musculoskeletal system. Clinical guidelines recommend biological therapies after conventional synthetic DMARDs (csDMARDs) inadequate response or intolerance. However, limited information has been published about biological therapies sequencing in clinical practice in terms of effectiveness and survival. Manhattan study aims to describe the effectiveness of guselkumab (GUS) or a second-line TNF inhibitor (TNFi) after receiving a first-line TNFi.
Methods: Manhattan (CNTO1959PSA4009) is an ongoing ambispective, observational cohort, study of patients with PsA in 34 hospitals in Spain. The study is evaluating effectiveness, persistence and tolerability of second-line GUS or TNFi patients after receiving a first-line TNFi treatment. An interim analysis was performed 10 months after the inclusion of the first patient. Sixty-nine participants (GUS n=36, TNFi n=33) had data available at week 12 and 56.5% (GUS n=25, TNFi n=14) at week 24, the maximum follow-up period in this analysis.
Results: Baseline clinical and demographic characteristics were described among all included patients for GUS and TNFi group, respectively. The mean age at PsA diagnosis was 42.8 for both groups, similar mean BMI was described (27.6 kg/m2 GUS and 29.3 kg/m2 TNFi) with 55.6% and 45.5% of females in GUS and TNFi group, respectively. The predominance of PsA characteristics were described for GUS and TNFi respectively: polyarticular PsA (63.9%, 78.1%); oligoarticular PsA (30.6%, 21.2%); mean Tender Joint Count (TJC) (5.7, 4.8); mean Swollen Joint Count (SJC) (3.5, 2.9); enthesitis (25.0%, 34.4%); dactylitis (19.4%, 12.5%); nail psoriasis (38.9%, 36.4%) and active psoriasis (66.7%, 45.5%). Concomitant csDMARDs at second-line were used in 38.9% of GUS patients and 51.5% of TNFi patients.
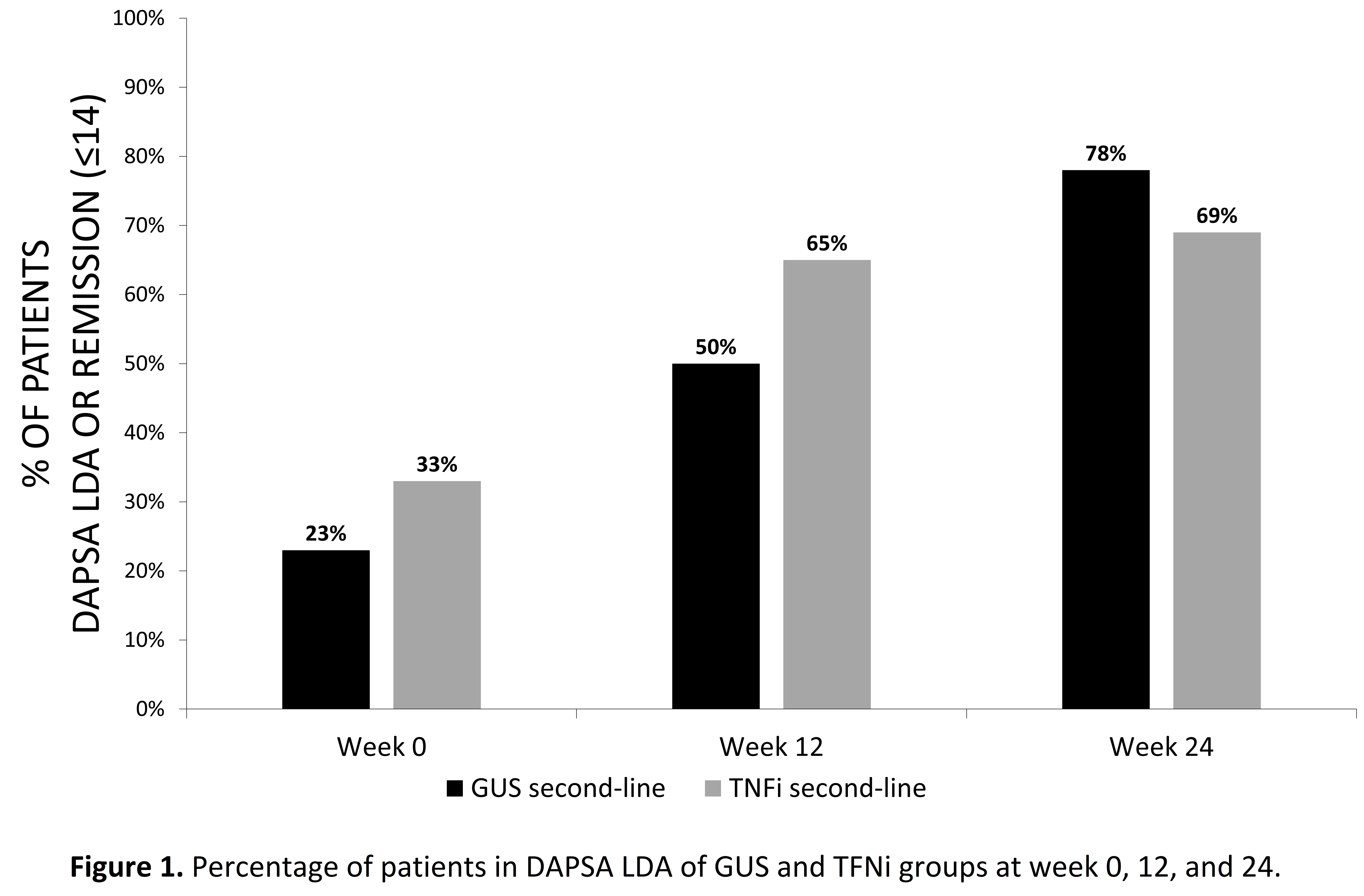
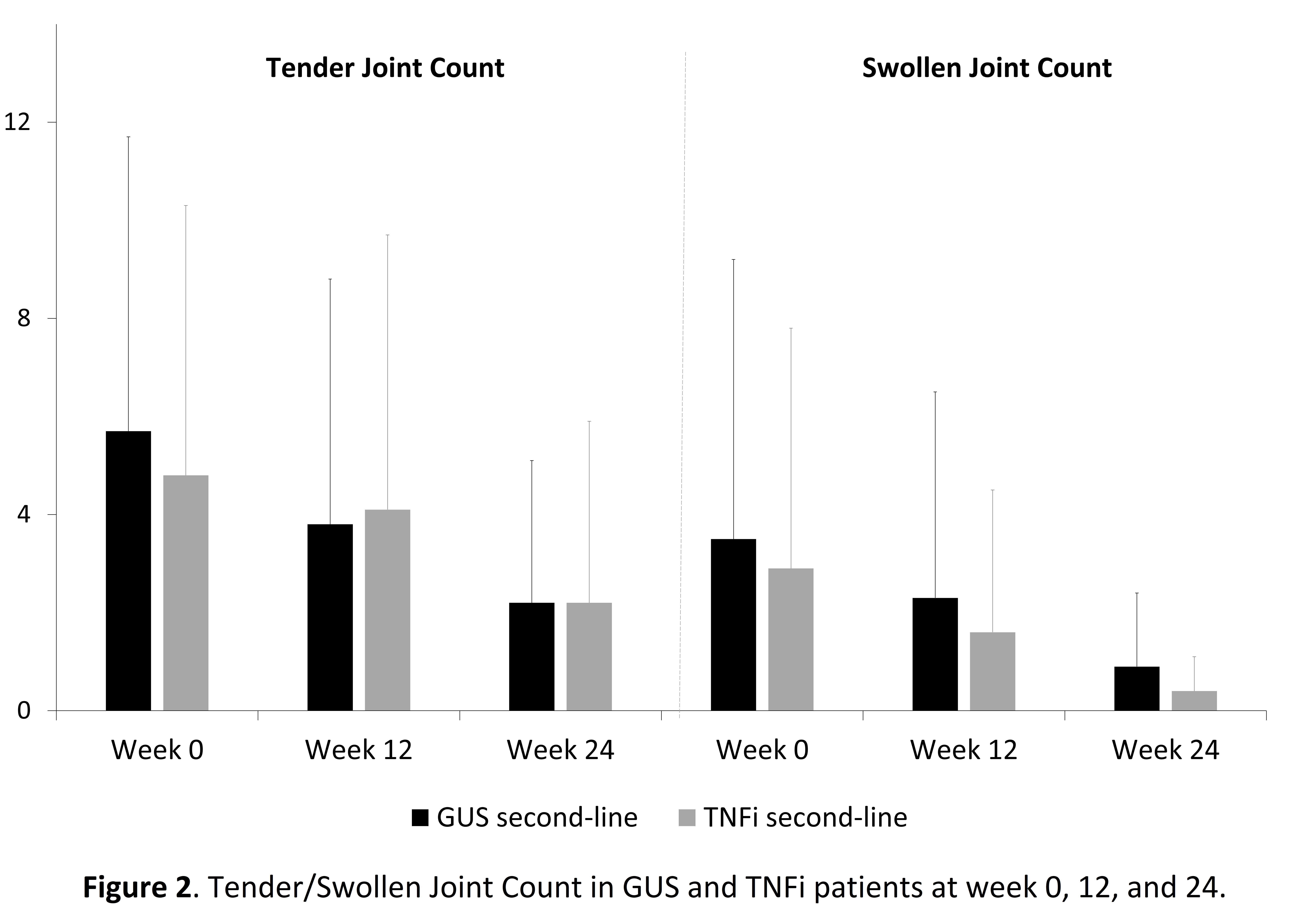
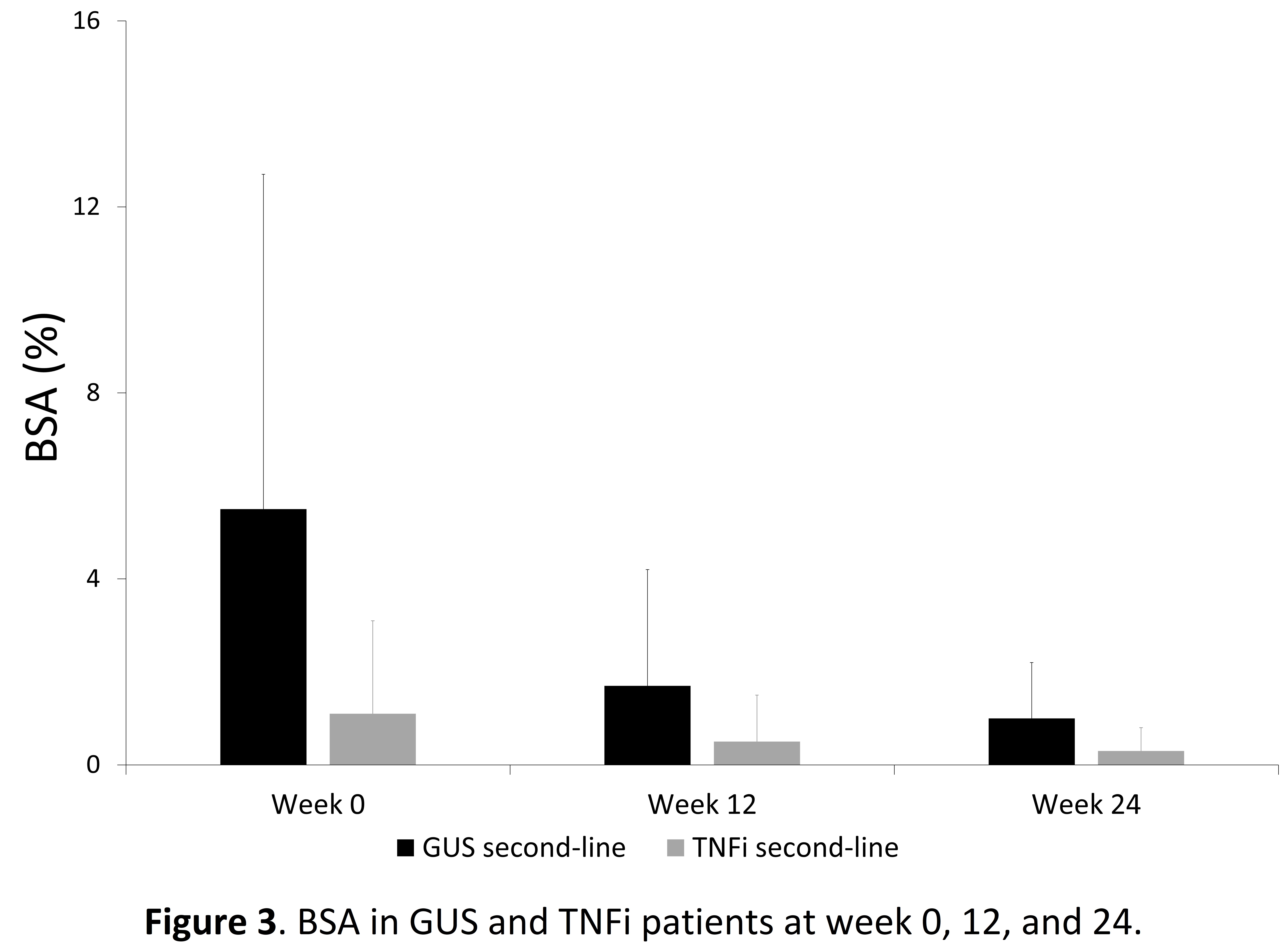
The percentage of patients with Disease Activity in Psoriatic Arthritis (DAPSA) Low Disease Activity (LDA) (≤ 14) increased over the time in both groups. After 24 weeks, 77.8% GUS patients and 69.2% TNFi patients were in DAPSA LDA (Figure 1). Accordingly, number of TJC and SJC were decreased over the time in both groups (Figure 2). Despite the baseline mean Body Surface Area (BSA) of 5.5% for GUS patients (Figure 3), 86.2% of patients had BSA ≤ 3% at week 12.
After 24 weeks, 95.7% of patients were still treated with GUS (1 patient suspended for patient desire) and 78.3% with TNFi (3 patient suspended due to adverse events and 1 due to primary failure).
One adverse drug reaction (ADR) was reported for GUS patients (headache) and four for TNFi patients (upper respiratory tract infection, syncope, alopecia, and skin reaction). No severe ADR was reported.
Conclusion: Among patients included in this interim analysis, DAPSA LDA was reached similarly after 24 weeks GUS or TNFi treatment whereas the probability of persistence to treatment was slightly higher in the GUS group.
To cite this abstract in AMA style:
González Molina M, Marín Huertas C, Joven-Ibáñez B, de Prado L, Mateo L, Llanes Gómez M, Hernández del Río A, Blanco-Morales E, Malavé Calzada J, Sallés Lizarzaburu M, Ros-Vilamajó I, Steiner M, CERVANTES PEREZ E, Hernández-Hernández V, Castro Oreiro S, URRUTICOECHEA A, Orpinell Palacio L, Campos Esteban J, Veroz González R, Camacho Alcázar O, Moreno-Gil M, Aragón Díez A, Moreno-Ramos M, Maceiras Pan F, Díaz-Castroverde S, Muñoz Fernández S, Pinto Tasende J, Ramírez García J. Manhattan Study: Observational, Ambispective Study to Describe Persistence and Effectiveness of a Second-line Guselkumab or TNF Inhibitors After First-line TNF Inhibitors for the Treatment of Active Psoriatic Arthritis in Spain [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/manhattan-study-observational-ambispective-study-to-describe-persistence-and-effectiveness-of-a-second-line-guselkumab-or-tnf-inhibitors-after-first-line-tnf-inhibitors-for-the-treatment-of-active-p/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/manhattan-study-observational-ambispective-study-to-describe-persistence-and-effectiveness-of-a-second-line-guselkumab-or-tnf-inhibitors-after-first-line-tnf-inhibitors-for-the-treatment-of-active-p/