Session Information
Date: Sunday, October 21, 2018
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Endothelial proliferation is a key finding in antiphospholipid antibody (aPL)-positive patients with microthrombosis. The mTOR pathway plays a role in the endothelial proliferation leading to aPL-related nephropathy; however the role of mTOR pathway is not well determined in patients with livedo, which is purple discoloration of the skin due to reactive dilation of peripheral dermal venules triggered by reduced blood flow in the center. Thus, our primary objective was to investigate mTOR pathway activation in the skin biopsies of aPL-positive patients with livedo reticularis/racemosa; secondly, we investigated mTOR activation in peripheral blood mononuclear cells (PBMC).
Methods: This ongoing cross-sectional study includes patients with livedo reticularis/racemosa: a) those with persistent aPL-positivity (LA, aCL IgG/M ≥ 40U, and/or aβ2GPI IgG/M ≥ 40U) with/without systemic lupus erythematosus (SLE); and b) aPL-negative SLE patients as a control group. We collect demographics and aPL-related history. We perform two 5-mm skin biopsies on each patient, one from the erythematous-violaceous (lesional) and the second from a non-violaceous area (central). Specimens are stained for phosphorylated S6 ribosomal protein (P-S6RP) as a marker of mTOR complex-1 activity;:;. We also collect PBMCs from aPL-positive patients to examine mTOR expression in monocytes assessed by flow cytometry. Unstimulated and lipopolysaccharide (LPS)-stimulated measurements are used to calculate the fold change in mTOR activation. Mann-Whitney-U test is used to compare the fold change between aPL-positive patients and sex- and age-matched healthy controls.
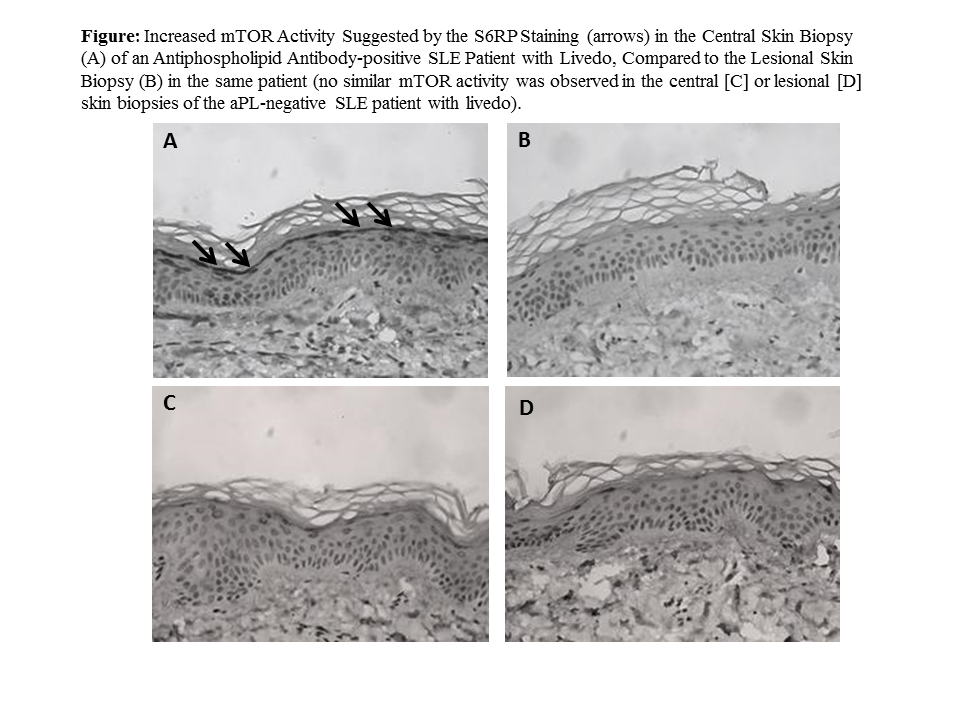
Results: As of May 2018, three aPL-positive SLE patients (all female and Caucasian; mean age: 40±11.2y; and 2 met APS clinical classification criteria) and one aPL-negative SLE control were enrolled. In all aPL-positive patients, but not in aPL-negative SLE control, there was increased mTOR activity in the non-violoaceous area (central) of the skin (epidermis), compared to erythematous-violaceous (lesional) skin (Figure). Unstimulated, LPS-stimulated, and fold change mTOR measurements were not different in monocytes of aPL-positive patients, compared to the healthy control group (p: 0.57, p: 0.73, and p: 0.56, respectively).
Conclusion: Based on the preliminary results of our ongoing study, we found increased mTOR activity in the center of livedoid lesions of aPL-positive patients with SLE, compared to an aPL-negative SLE patient. However, there was no systemic mTOR activation in monocytes. Given the vascular stenosis occurring in arterioles located in the center leading to livedo at the periphery, our findings are consistent with the pathophysiology of livedoid lesions. Further analysis with increased number of patients will determine the clinical relevance of our findings.
To cite this abstract in AMA style:
Sevim E, Siddique S, Chyou S, Shipman WD, Eugenio-Fernandez I, Badger A, O`Shea O, Zuily S, Harp J, Magro C, Alpan O, Lu TT, Erkan D. Mammalian Target of Rapamycin (mTOR) Pathway Assessment in Antiphospholipid Antibody Positive Patients with Livedo Reticularis/racemosa [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/mammalian-target-of-rapamycin-mtor-pathway-assessment-in-antiphospholipid-antibody-positive-patients-with-livedo-reticularis-racemosa/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mammalian-target-of-rapamycin-mtor-pathway-assessment-in-antiphospholipid-antibody-positive-patients-with-livedo-reticularis-racemosa/