Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment I: Psoriatic Arthritis (0504–0508)
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
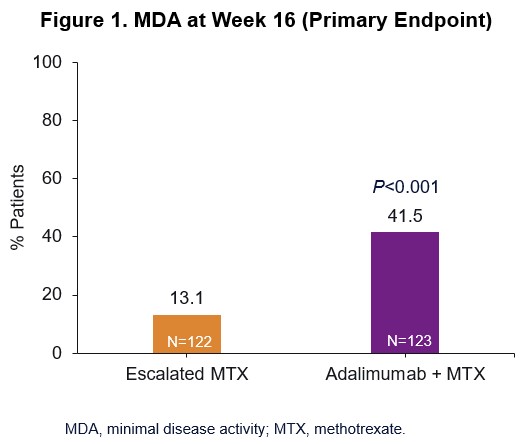
Background/Purpose: Minimal Disease Activity (MDA) is suggested as an appropriate treat-to-target outcome for patients (pts) with PsA. Results from Part 1 of the CONTROL study demonstrated that the introduction of adalimumab (ADA) after an initial course of MTX 15 mg resulted in significantly higher MDA rates at week (wk) 16 compared with escalating MTX dose (Fig 1).1 Here we present results from Part 2 of the CONTROL study, in which therapy was either maintained or modified based upon MDA response at wk 16.
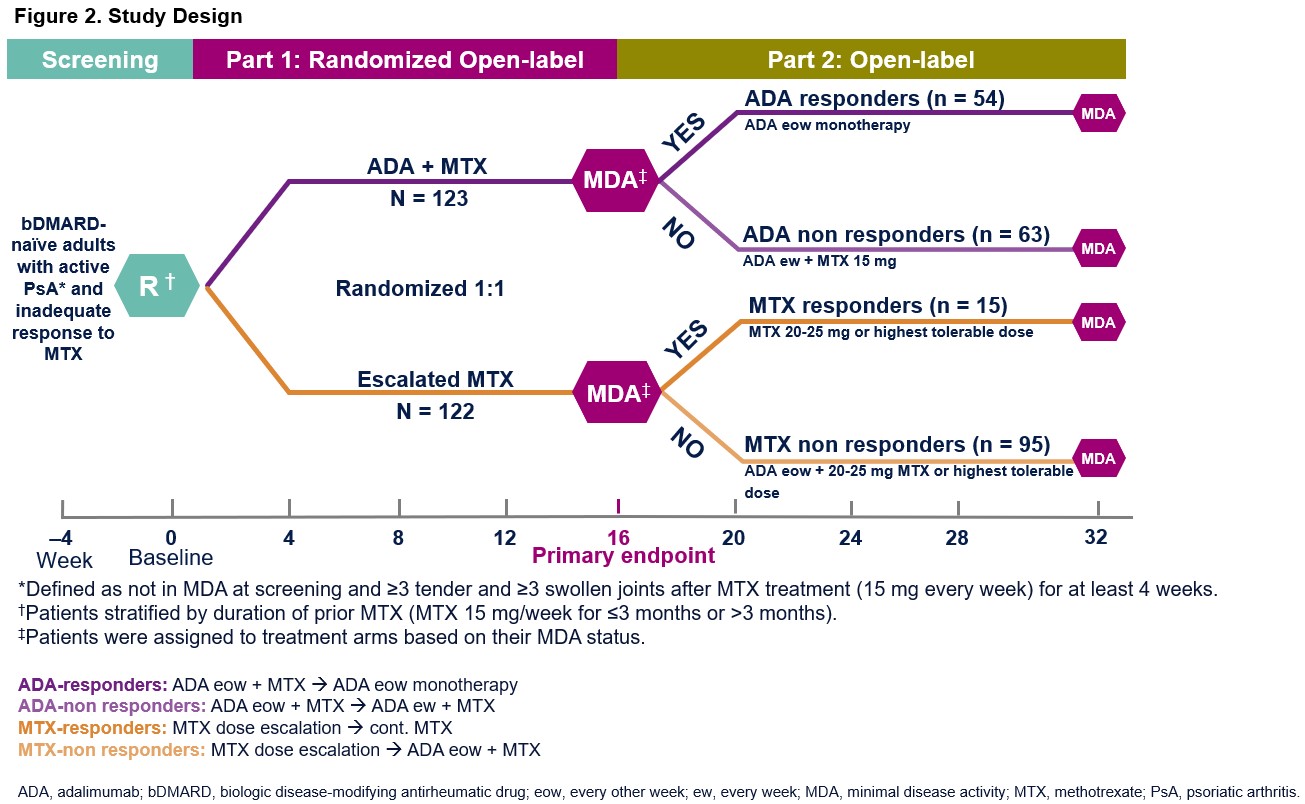
Methods: The open-label, 2-part CONTROL study enrolled bDMARD-naive adult pts with active PsA (not in MDA at screening and ≥3 tender and ≥3 swollen joints) despite MTX 15 mg every wk (ew) for ≥4 wks.1 During Part 1, pts were randomized to ADA 40 mg every other wk (eow) + MTX 15 mg (ADA + MTX) or escalated MTX to 20–25 mg ew or highest tolerable dose for 16 wks (Fig 2). In Part 2 (wk 16 through 32), pts initially randomized to ADA + MTX who achieved MDA at wk 16 (ADA responders) discontinued MTX and continued ADA 40 mg eow as monotherapy; those initially randomized to ADA + MTX who did not achieve MDA (ADA‑non responders) escalated therapy to ADA 40 mg ew + MTX 15 mg ew. For pts initially randomized to escalated MTX, if MDA was achieved at wk 16 (MTX responders), weekly MTX (20-25 mg or highest tolerable dose ew) was continued; if MDA was not achieved (MTX non-responders), pts were switched to ADA 40 mg eow + MTX 20-25 mg or highest tolerable dose ew. Data are reported as observed.
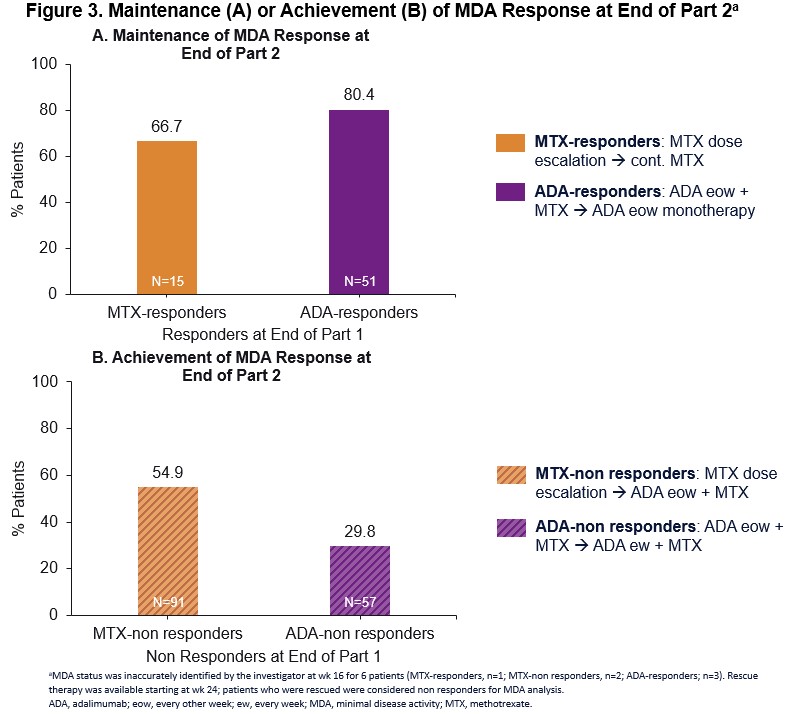
Results: At wk 16, 54 and 15 pts achieved MDA on ADA + MTX or escalated MTX (ADA responders and MTX responders, respectively) (Fig 2). There were 63 ADA-non responders and 95 MTX-non responders who did not achieve MDA at wk 16. In the responder groups, 80% of ADA responders were in MDA state at the end of Part 2 after switching from ADA + MTX to ADA monotherapy; whereas 67% of MTX responders were in MDA after maintaining their escalated MTX therapy during Part 2 (Fig 3A). Among MTX-non responders who switched from escalated MTX to ADA eow + MTX in Part 2, 55% achieved MDA at the end of Part 2 (Fig 3B). Thirty percent of ADA-non responders who did not respond to ADA eow + MTX in Part 1 were able to achieve MDA following escalation of therapy to ADA ew + MTX (Fig 3B). In Part 2, the proportion of pts with treatment-emergent adverse events (AE) was as follows: ADA responders: 44.4% (24/54); ADA-non responders: 66.7% (42/63); MTX responders: 33.3% (5/15); MTX-non responders: 56.8% (54/95). Serious AEs were reported infrequently (< 5%) across all groups. Two malignancies were reported: one each in the ADA responder and ADA-non responder groups. There were no opportunistic infections, tuberculosis, or deaths or new safety signals identified in Part 2.
Conclusion: Patients who achieved MDA response in Part 1 generally maintained efficacy through Part 2 despite a reduction in or maintenance of current therapy, with numerically higher proportions of patients achieving MDA response in ADA responders vs MTX responders at wk 32. For patients who did not achieve MDA after 16 wks in Part 1, modification of therapy by addition or escalation of ADA resulted in increased proportions of patients achieving MDA at the end of Part 2. The safety profile of ADA was consistent with the known safety profile.
REF
- Coates et al. Ann Rheum Dis 2020;79(s1):33
To cite this abstract in AMA style:
Mease P, Conaghan P, Tillett W, D'Agostino M, Rahman P, Behrens F, Blondell E, Bu X, Chen L, Kapoor M, Coates L. Maintenance or Achievement of Minimal Disease Activity Following Therapy Optimization with Adalimumab or Methotrexate in Patients with Psoriatic Arthritis: Results from Part 2 of a Randomized, Open-Label Phase 4 Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/maintenance-or-achievement-of-minimal-disease-activity-following-therapy-optimization-with-adalimumab-or-methotrexate-in-patients-with-psoriatic-arthritis-results-from-part-2-of-a-randomized-open-la/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/maintenance-or-achievement-of-minimal-disease-activity-following-therapy-optimization-with-adalimumab-or-methotrexate-in-patients-with-psoriatic-arthritis-results-from-part-2-of-a-randomized-open-la/