Session Information
Date: Sunday, November 13, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) is associated with loss of physical function, work disability, and decreased quality of life. Early treatment with certolizumab pegol (CZP) in combination with optimized MTX improves physical function1 and workplace and household productivity2 in DMARD-naïve, early RA patients (pts) after 1 year. Here, we report the impact on physical function, and workplace and household productivity of 2 years of CZP+MTX therapy in early, active RA pts with severe progressive disease.
Methods: Pts treated with CZP (200 mg Q2W+MTX) or placebo (PBO+MTX) from C-EARLY Period 1 (NCT01519791),1 who achieved sustained low disease activity (sLDA; DAS28[ESR] ≤3.2 at both Weeks [Wks] 40 and 52) entered Period 2 (NCT01521923),3 a randomized, double-blind dose withdrawal study. At Wk 52, CZP-treated pts in sLDA were randomized 2:3:2 to CZP standard dose (200 mg Q2W+MTX), reduced dose-frequency (200 mg Q4W+MTX), or CZP stopped (PBO+MTX). The percentage of pts achieving normative physical function (HAQ-DI ≤0.5) at Wks 52 and 104; employment status, workplace and household productivity (Work Productivity Survey, WPS),4 and need for assistance at baseline (BL), Wk 52, and Wk 104 are reported. Missing values were imputed using last observation carried forward (LOCF).
Results: 84, 126, 79 pts in the CZP standard, reduced dose-frequency, and CZP stopped groups, respectively, comprise the full analysis set. Pt characteristics and employment status at BL and Wk 52 were generally similar across all groups. The proportion of pts achieving normative physical function was generally maintained from Wk 52 to Wk 104 in CZP standard and reduced dose-frequency pts. Fewer pts who stopped CZP maintained normative physical function to Wk 104. Employed pts continuing CZP treatment (standard and reduced dose-frequency) maintained improvements achieved at Wk 52 in workplace and household productivity to Wk 104, with some worsening seen in CZP stopped pts. Similar trends were observed in household productivity. At Wk 104, more pts who stopped CZP experienced the need for regular assistance with usual activities than pts continuing CZP treatment (standard and reduced dose-frequency) (Table).
Conclusion: Pts who continued CZP treatment at the standard or reduced dose-frequency maintained the initial improvements over the second year in normative physical function, and workplace and household productivity, and also maintained the reduced need for regular assistance from a relative or friend. A deterioration was seen from Wks 52–104 in pts who stopped CZP after 1 year. References: 1. Emery P. Ann Rheum Dis 2016;doi:10.1136/annrheumdis-2015-209057; 2. Emery P. Ann Rheum Dis 2015;74(S2):712; 3. Emery P. Ann Rheum Dis 2016;75(S2):143; 4. Osterhaus J. Arthritis Res Ther 2009;11:R73 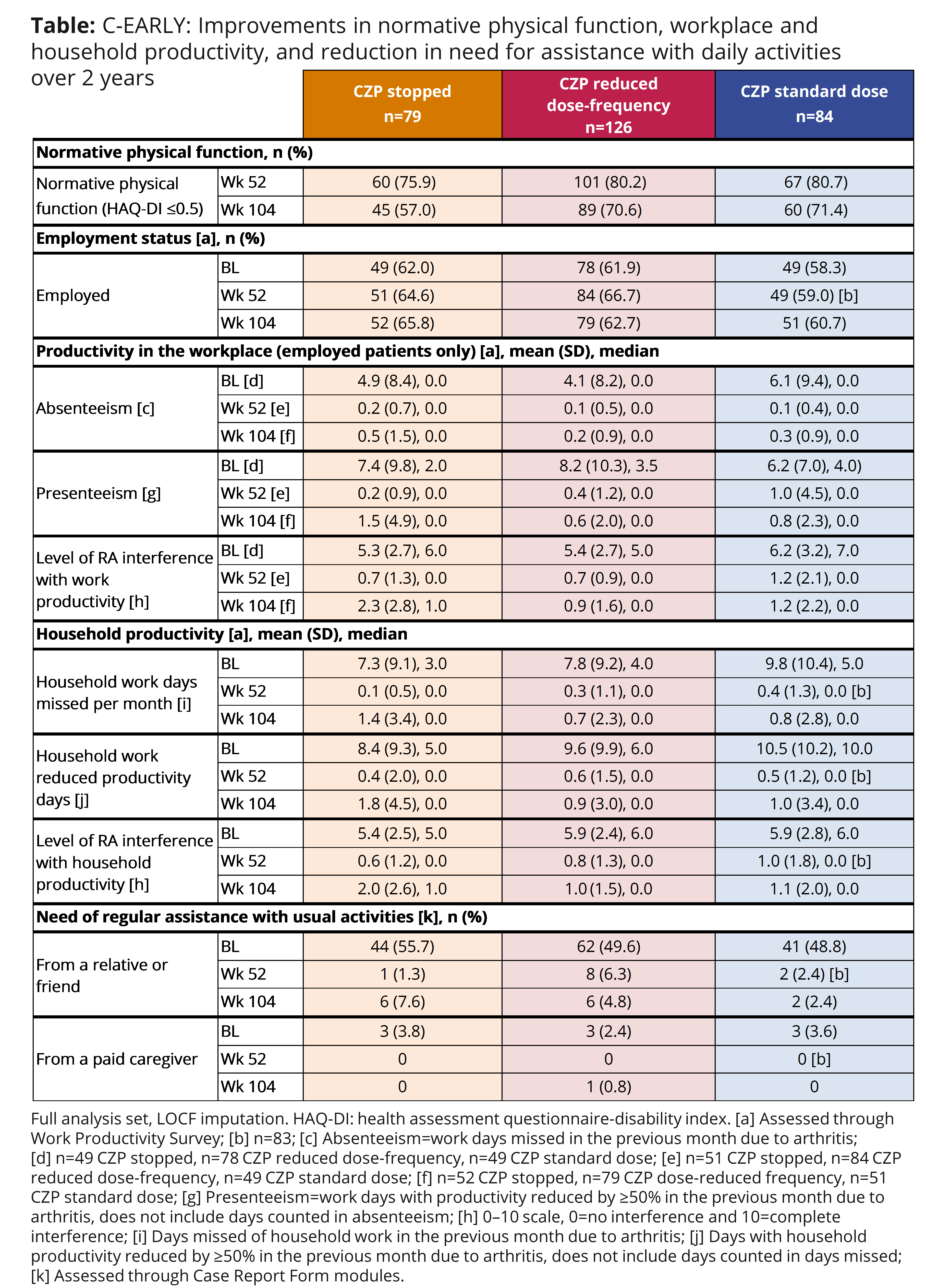
To cite this abstract in AMA style:
Bingham C III, Emery P, Weinblatt M, Burmester GR, Furst DE, Mariette X, van Vollenhoven R, VanLunen B, Purcaru O, Bykerk VP. Maintenance of Improvements in Patients’ Physical Function, Workplace and Household Productivity, and Reduction in Caregiver Burden with 2 Years of Certolizumab Pegol Treatment in DMARD-Naive, Early RA Patients with Severe Progressive Disease [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/maintenance-of-improvements-in-patients-physical-function-workplace-and-household-productivity-and-reduction-in-caregiver-burden-with-2-years-of-certolizumab-pegol-treatment-in-dmard-naive-early/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/maintenance-of-improvements-in-patients-physical-function-workplace-and-household-productivity-and-reduction-in-caregiver-burden-with-2-years-of-certolizumab-pegol-treatment-in-dmard-naive-early/
