Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Deucravacitinib is a first-in-class, oral, selective, allosteric tyrosine kinase 2 inhibitor approved in multiple countries for the treatment of adults with plaque psoriasis. A 48-week, double-blind, phase 2 trial (NCT03252587) in patients with SLE showed that deucravacitinib demonstrated efficacy across multiple endpoints compared with placebo, including the primary endpoint of Systemic Lupus Erythematosus Responder Index 4 (SRI[4]) at week 32 and the secondary endpoint of LLDAS at week 48.1 Achievement and maintenance of LLDAS is associated with reduced risk of SLE-associated organ damage and mortality.2 Here, we further investigate LLDAS outcomes with deucravacitinib through post hoc analyses assessing time to first LLDAS response and cumulative time in LLDAS.
Methods: Patients with active SLE (N=363) were randomized 1:1:1:1 to placebo or deucravacitinib 3 mg twice daily (BID), 6 mg BID, or 12 mg once daily (QD). Exploratory outcomes included median time to first LLDAS response and the percentage of patients who achieved LLDAS for ≥ 3 or ≥ 5 consecutive visits and the percentage of patients who achieved LLDAS for ≥ 20% or ≥ 50% of the time through week 48, via logistic regression. Odds ratios and nominal P values for each treatment group compared with the placebo group were provided.
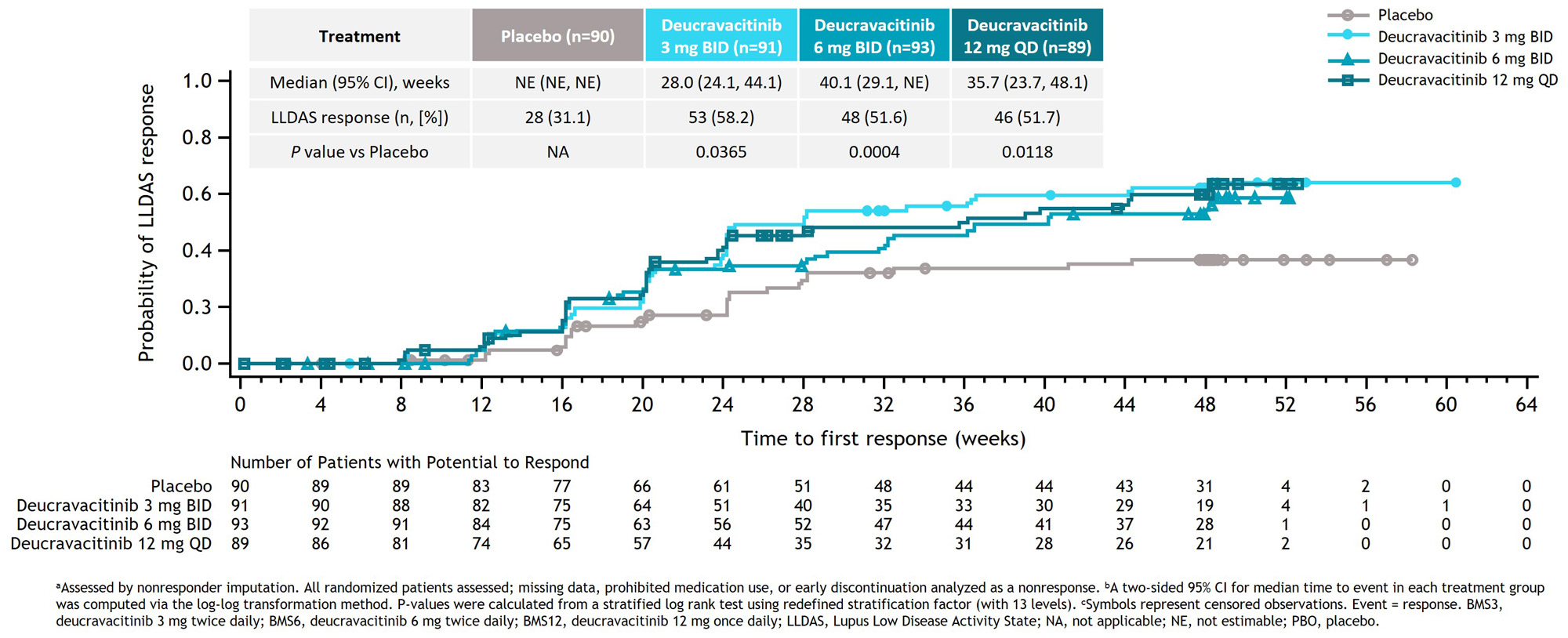
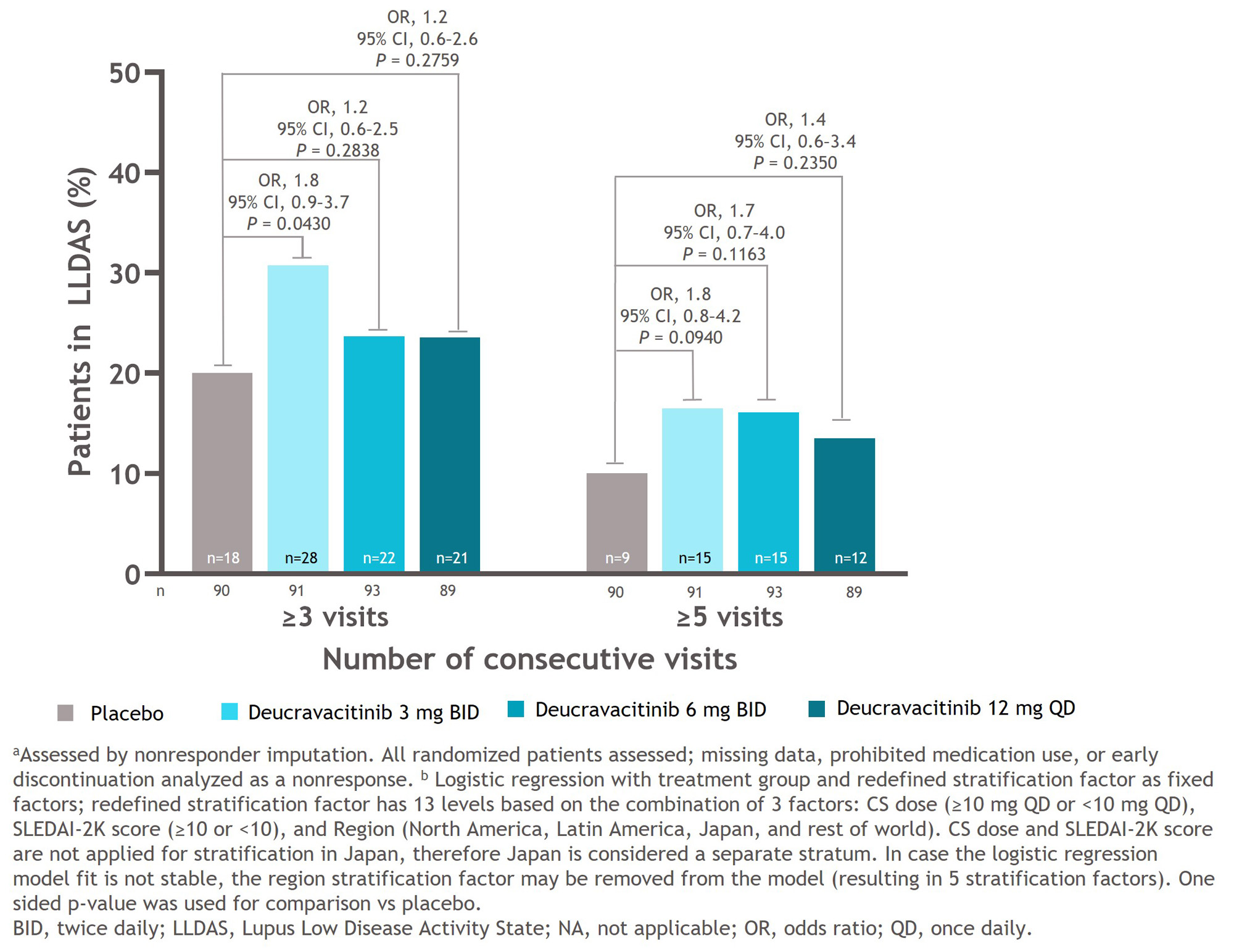
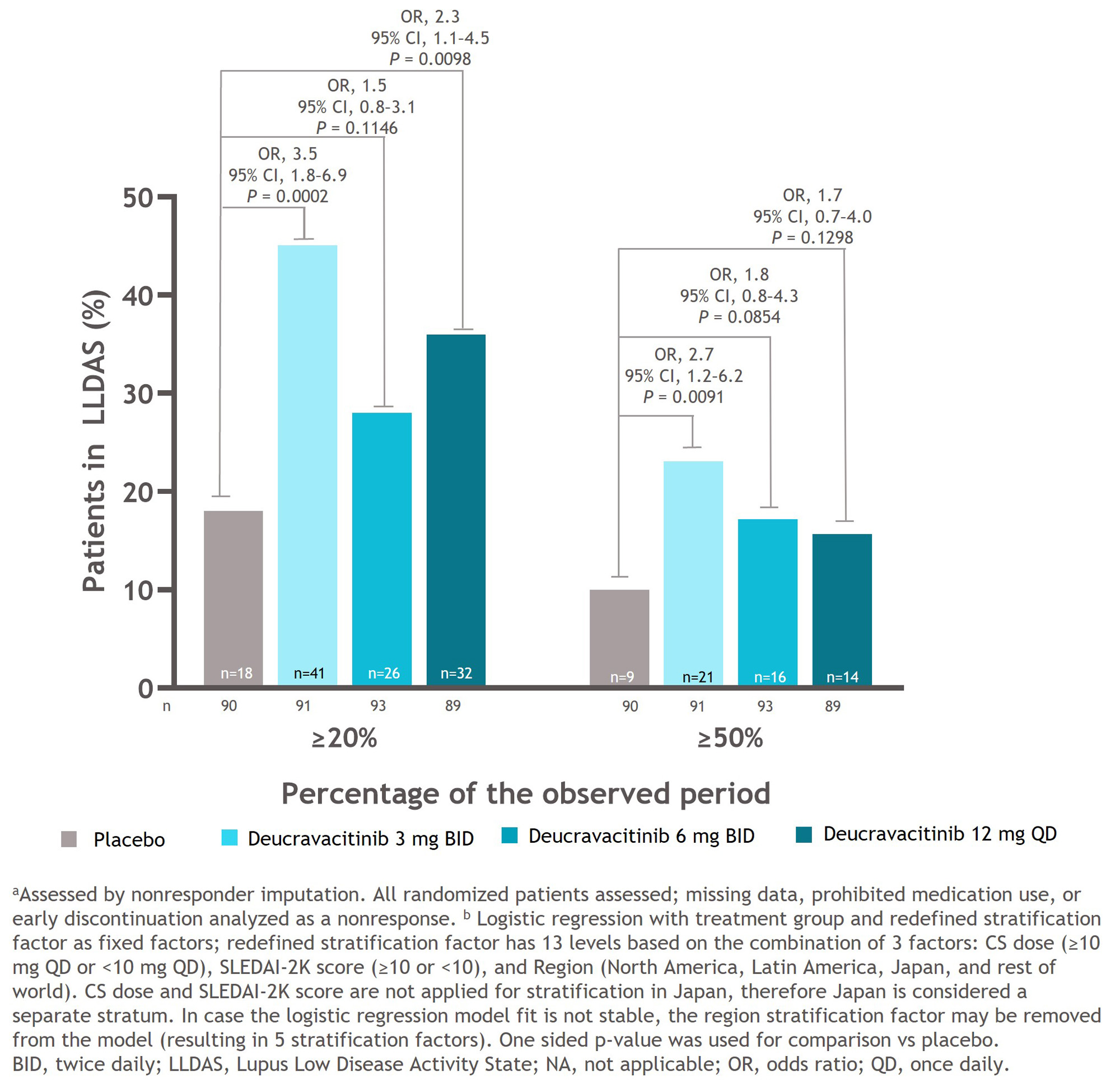
Results: Median time to first LLDAS response was significantly shorter in all deucravacitinib groups compared with the placebo group (3 mg BID, 28.0 weeks: P = 0.0365 vs placebo; 6 mg BID, 40.1 weeks: P = 0.0004 vs placebo; 12 mg QD, 35.7 weeks: P = 0.0118 vs placebo). Median time to first LLDAS response was not estimable in the placebo group because 50% of the patients had not yet achieved an LLDAS response (Figure 1). The deucravacitinib 3 mg BID group had a significantly higher percentage of patients who achieved LLDAS for ≥ 3 visits compared with the placebo group (OR, 1.8; 95% CI, 0.9–3.7, P = 0.0430). A higher percentage of patients treated with any dose of deucravacitinib attained LLDAS for ≥ 3 or ≥ 5 visits compared with patients receiving placebo (Figure 2). A higher percentage of patients treated with deucravacitinib attained LLDAS for ≥ 20% and ≥ 50% of cumulative observed time compared with patients receiving placebo (Figure 3). The deucravacitinib 3 mg BID and 12 mg QD groups had significantly higher percentages of patients who achieved LLDAS for ≥ 20% of cumulative time compared with placebo (3 mg BID: OR, 3.5; 95% CI, 1.8–6.9; P = 0.0002; 12 mg QD: OR, 2.3; 95% CI, 1.1–4.5; P = 0.0098), and the 3 mg BID group also had a significantly higher percentage of patients who achieved LLDAS for ≥ 50% of cumulative time (3 mg BID: OR, 2.7; 95% CI, 1.2–6.2; P = 0.0091).
Conclusion: Patients treated with deucravacitinib achieved LLDAS response earlier and demonstrated more sustained and cumulative LLDAS responses compared with patients receiving placebo. Taken together, these data suggest that deucravacitinib may contribute to better long-term prospects for patients with SLE.
Reference:
1. Morand E, et al. Arthritis Rheumatol 2023;75:242–252.
2. Petri M, Magder LS. Arthritis Rheumatol 2018; 70(11); 1790–1795.
To cite this abstract in AMA style:
Morand E, Hobar C, Pomponi S, Koti R, Wegman T, van Vollenhoven R. Lupus Low Disease Activity State (LLDAS) Achievement with Deucravacitinib, an Oral, Selective, Allosteric Tyrosine Kinase 2 Inhibitor, in a Phase 2 Trial in SLE [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/lupus-low-disease-activity-state-lldas-achievement-with-deucravacitinib-an-oral-selective-allosteric-tyrosine-kinase-2-inhibitor-in-a-phase-2-trial-in-sle/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lupus-low-disease-activity-state-lldas-achievement-with-deucravacitinib-an-oral-selective-allosteric-tyrosine-kinase-2-inhibitor-in-a-phase-2-trial-in-sle/