Session Information
Date: Sunday, November 7, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster II: Manifestations (0855–0896)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Mortality in patients with systemic lupus erythematosus (SLE) is high compared to the general population. Attainment of the Lupus Low Disease Activity State (LLDAS) is validated as protective against adverse outcomes including organ damage accrual and flare. We examined whether LLDAS attainment provided protection against mortality in patients with SLE. In addition, we examined remission (Definitions for Remission in SLE (DORIS) definitions).
Methods: Data from a 13-country longitudinal SLE cohort on patients meeting either ACR or SLICC criteria were collected between 2013 and 2020 using standard case report files. LLDAS was defined as in Golder et al., 2019 (SLEDAI<4, no new activity, PGA <1, pred <7.5 mg/d, antimalarials (AM) and immunosuppressants (IS) allowed. Remission definitions were as in Vollenhoven et al, 2016 included clinical remission on treatment (CROT: clinical SLEDAI=0, AM and IS allowed, prednisolone <5 mg/d), and a variation disallowing glucocorticoid (CROT-off-steroid). Longitudinal associations of mortality were examined using survival (Cox regression) analysis.
Results: The study cohort included 4,020 SLE patients followed over a median of 2.6 years [IQR: 1.0 to 5.1] (Table 1). Ninety-one patients died during the study observation period; the crude mortality rate was ~ 7/1000 person-years. About 68% of deaths were related to infections; ~49% were related to SLE; ~24% related to cardiovascular causes and about 13% related to malignancy.
Disease activity and organ damage were strongly and independently associated with mortality. A 1 point increase in SLEDAI-2K was associated with an increased risk of mortality of 15% after accounting for confounders (adjusted HR: 1.15 (1.07, 1.23), p< 0.001). A 0.3-increase in PGA increased the risk of mortality by 33% (adjusted HR = 1.33 (1.13, 1.56), p=0.001). Every 1 unit increase in SDI score increased the mortality hazard by 53% (adjusted HR = 1.53 (1.38, 1.70), p< 0.001). While the associations of cumulative prednisolone exposure and flares with mortality were significant in univariable analysis, they attenuated in multivariable analysis.
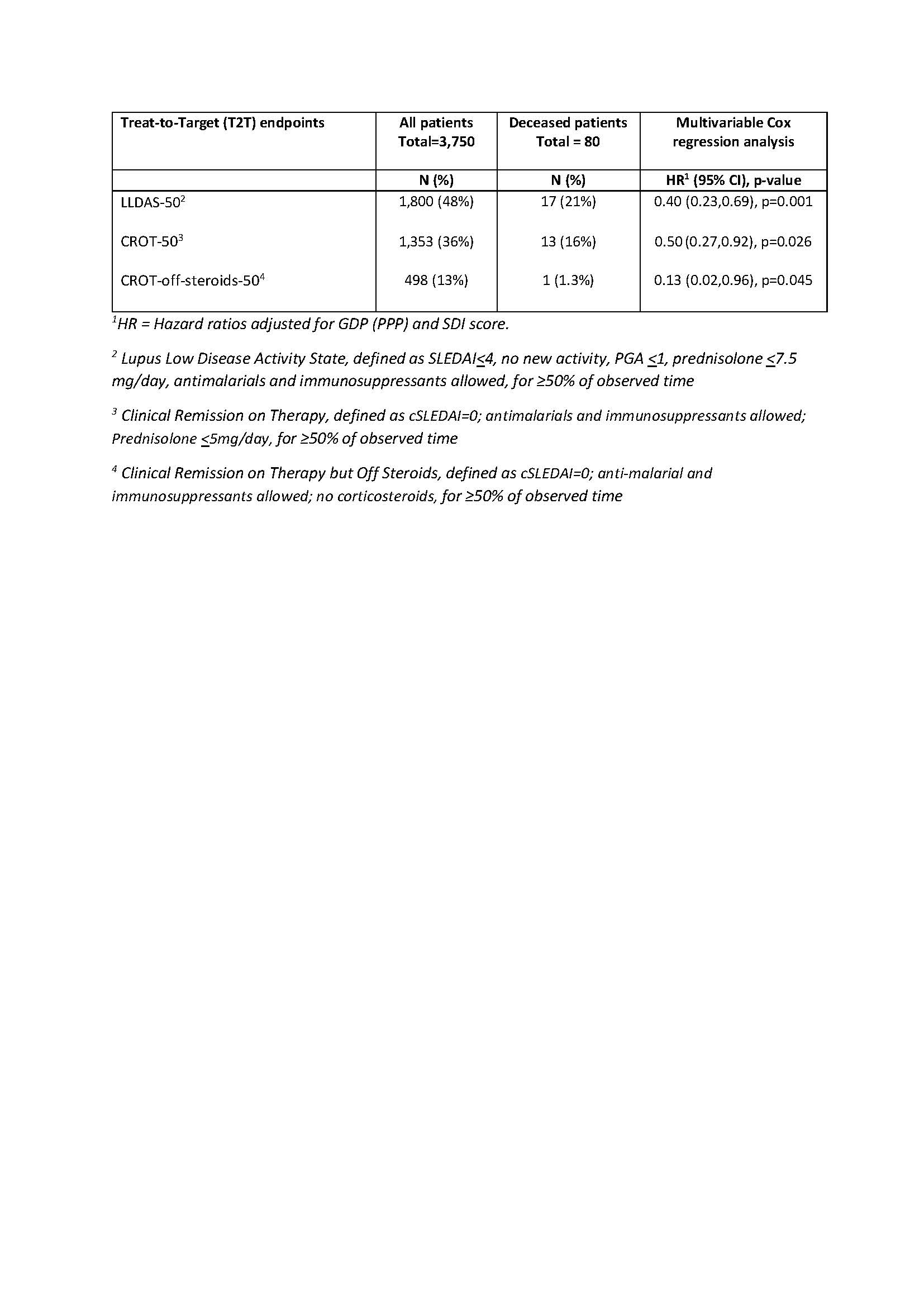
48% of all patients achieved ≥50% observed time in LLDAS (LLDAS-50) but only 21% of those who died (Table 2). LLDAS-50 reduced mortality by 60% (adjusted HR = 0.40 (95% CI: 0.23, 0.69) p< 0.001) (Table 2). Clinical remission on treatment >50% of observed time (CROT-50) was achieved in 36% of patients and reduced mortality by 50% (HR = 0.50 (0.27, 0.92), p=0.026). A greater reduction in mortality was observed in patients who spent ≥50% time on CROT-off-steroid (HR=0.13 (0.02,0.96), p=0.045), but this was only achieved in 13% of patients (Table 2).
Conclusion: The attainment of LLDAS and DORIS remission conferred significant protection against mortality in SLE. LLDAS was more attainable than remission. Compared to LLDAS, clinical remission on treatment was not more protective, while clinical remission off steroids was maximally protective. Steroid free remission should be the goal of treatment in SLE.
To cite this abstract in AMA style:
Kandane-Rathnayake R, Golder V, Louthrenoo W, CHEN Y, Cho J, Lateef A, Hamijoyo L, Luo S, Wu Y, Navarra S, Zamora L, Li Z, An Y, Sockalingam S, Katsumata Y, Harigai M, Hao Y, Zhang Z, Basnayake B, Chan M, Kikuchi J, Takeuchi T, Oon S, Bae S, O’Neill S, Goldblatt F, Gibson K, Ng K, Law A, Tugnet N, Kumar S, Tee M, Yu D, Karyekar C, Tanaka Y, Lau C, Nikpour M, Hoi A, Morand E. Lupus Low Disease Activity State Attainment Provides Significant Protection Against Mortality: A Multi-National, Longitudinal Observational Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/lupus-low-disease-activity-state-attainment-provides-significant-protection-against-mortality-a-multi-national-longitudinal-observational-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lupus-low-disease-activity-state-attainment-provides-significant-protection-against-mortality-a-multi-national-longitudinal-observational-study/