Session Information
Date: Monday, November 9, 2020
Title: SLE – Treatment Poster II
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: In the randomized, double-blind, phase 2b MUSE trial, anifrolumab reduced disease activity vs placebo across multiple endpoints in patients with moderately to severely active SLE.1 Here, we assess changes in disease activity and safety in patients after cessation of anifrolumab treatment during the follow-up period in MUSE.
Methods: Patients were randomized 1:1:1 to receive placebo or anifrolumab 300 or 1000 mg IV every 4 weeks (wks). The final study dose was at Wk 48, and key efficacy endpoints were assessed at Wk 52. After receiving the final dose of study medication, patients were required to complete a follow-up period during which visits were conducted every 4 wks (±7 days) (Figure 1). Disease activity (measured with SLEDAI-2K and BILAG-2004 global scores, and Cutaneous Lupus Erythematosus Disease Area and Severity Index [CLASI] activity score), flares (defined as either ≥1 new BILAG-2004 A or ≥2 new BILAG-2004 B domain scores), adverse events (AEs), and 21-gene type I IFN gene signatures (IFNGS) were assessed. Disease activity scores, flares, and IFNGS were assessed over 8 wks from Wk 52 through Wk 60, and safety was evaluated over 12 wks from Wk 48 through Wk 60 or upon study discontinuation.
Results: Of 305 patients randomized in MUSE, 229 completed the last study visit (Wk 52): 86, 75, and 68 in the anifrolumab 300-mg, 1000-mg, and placebo groups, respectively. From Wk 52 to the end of the follow-up period (Wk 60), mean SLEDAI-2K global scores increased in patients ceasing treatment of anifrolumab 300 mg (mean change: 0.7) and 1000 mg (0.3), but not placebo (−0.1) (Table 1). A numeric worsening was also observed in mean BILAG-2004 global scores in patients ceasing anifrolumab 300-mg treatment (mean change: 2.4) vs placebo (0.8); in the 1000-mg group, mean change was 0.7. Of the various domains, worsening activity scores were most frequently observed in the mucocutaneous and musculoskeletal domains. Overall, 15.2% and 6.7% of patients ceasing treatment of anifrolumab 300 or 1000 mg, respectively, had ≥1 BILAG flare from Wk 52 through Wk 60 vs 2.0% with placebo. Mean CLASI activity scores increased slightly from Wk 52 to Wk 60 across the anifrolumab 300-mg (mean change: 0.4), 1000-mg (0.4), and placebo groups (0.5). From Wk 52 to Wk 60, IFNGS expression increased more rapidly in the anifrolumab 300-mg group vs the 1000-mg group, with negligible changes in the placebo group (Figure 2). AEs during the 12-wk safety follow-up period were similar between the anifrolumab 300-mg and 1000-mg vs placebo groups (≥1 AE: 29.3% and 26.7% vs 24.8%; ≥1 serious AE: 3.0% and 3.8% vs 5.0%).
Conclusion: No new or unexpected safety findings were observed in the MUSE follow-up period. There was a larger numeric worsening of disease activity in patients ceasing anifrolumab compared with placebo. A rebound in IFNGS was observed in patients previously treated with anifrolumab; this effect was more apparent with 300 vs 1000 mg. An analysis is underway evaluating the relationship between changes in IFNGS and disease activity.
References
- Furie RA. Arthritis Rheumatol. 2017;69:376–86.
Writing assistance by Dominic Johnson, PhD (JK Associates Inc., a Fishawack Health Company).
This study was sponsored by AstraZeneca.
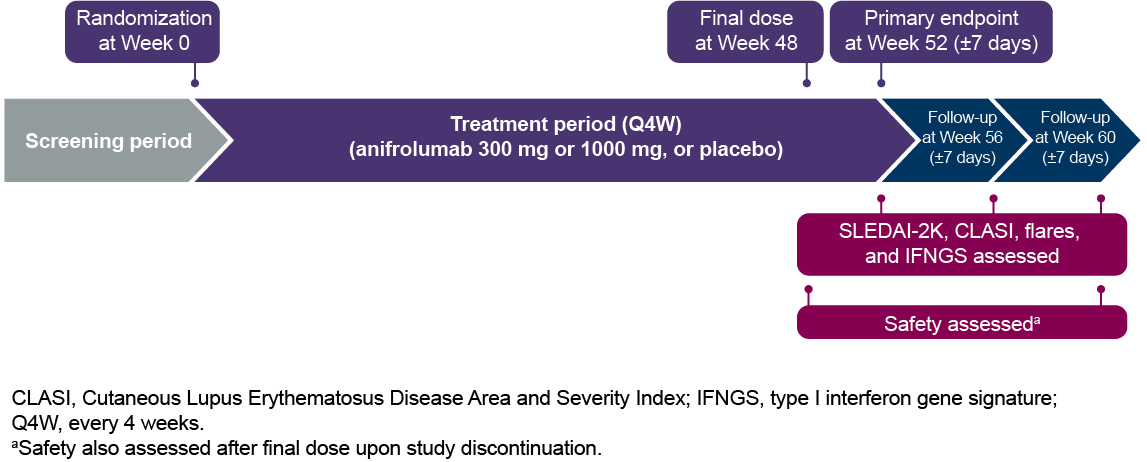 Figure 1. Trial Design for MUSE
Figure 1. Trial Design for MUSE
 Figure 2. Change in Neutralization Ratio of the 21-Gene Type I IFNGS From Start of the MUSE Trial to End of Follow-up (Week 60)
Figure 2. Change in Neutralization Ratio of the 21-Gene Type I IFNGS From Start of the MUSE Trial to End of Follow-up (Week 60)
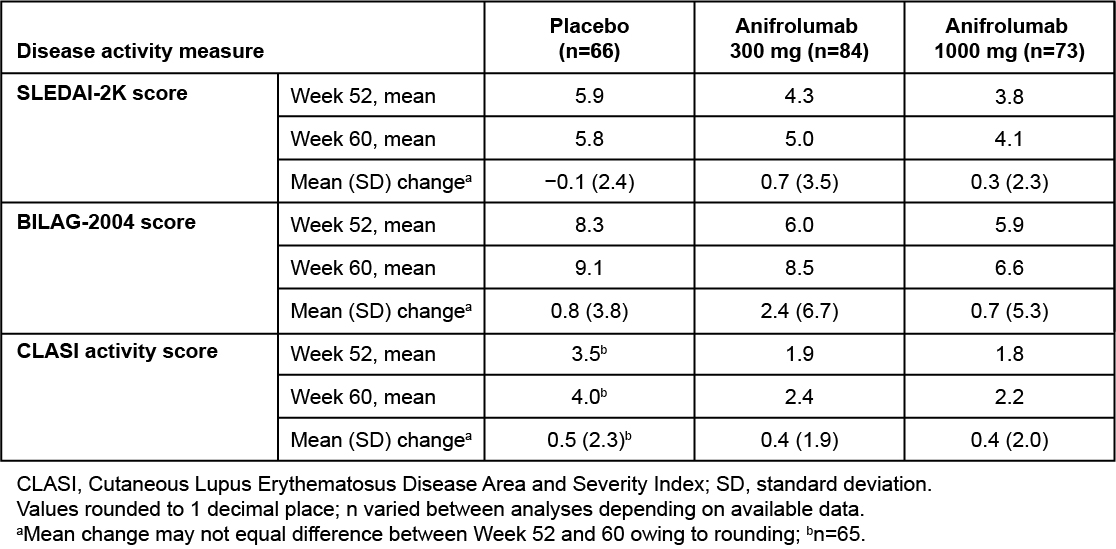 Table 1. Mean Change in SLEDAI-2K, BILAG-2004, and CLASI Activity Scores From MUSE Trial Efficacy Endpoint (Week 52) to End of Follow-up (Week 60)
Table 1. Mean Change in SLEDAI-2K, BILAG-2004, and CLASI Activity Scores From MUSE Trial Efficacy Endpoint (Week 52) to End of Follow-up (Week 60)
To cite this abstract in AMA style:
Furie R, Kalunian K, Merrill J, Abreu G, Tummala R. Lupus Disease Activity After Cessation of Anifrolumab Treatment During the Phase 2b MUSE Trial Follow-up Period [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/lupus-disease-activity-after-cessation-of-anifrolumab-treatment-during-the-phase-2b-muse-trial-follow-up-period/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lupus-disease-activity-after-cessation-of-anifrolumab-treatment-during-the-phase-2b-muse-trial-follow-up-period/
