Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Indomethacin is used to treat acute gouty arthritis and other acute pain conditions but, like other NSAIDs, is associated with dose-related gastrointestinal, cardiovascular, and renal complications. A US Food and Drug Administration Public Health Advisory recommended that physicians use NSAIDs at “the lowest effective dose for the shortest duration consistent with individual patient treatment goals.” A challenge with using existing NSAIDs at lower doses is achieving efficacy. New, submicron particle NSAIDs with enhanced absorption are under investigation to evaluate efficacy at lower doses than commercially available NSAIDs. We report the results of two phase 3 studies that evaluated investigational, lower-dose indomethacin submicron particle capsules compared with placebo in patients with acute pain following elective surgery.
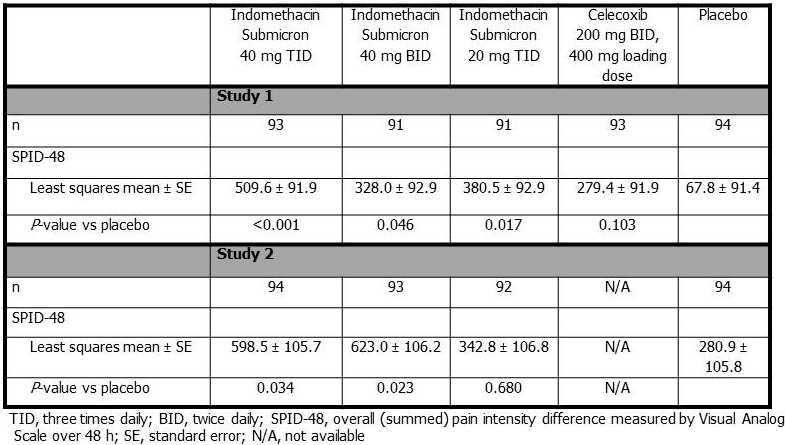
Methods: Two phase 3, multi-center, randomized, double-blind studies enrolled patients 18 to 65 years of age undergoing bunionectomy with osteotomy and internal fixation under regional anesthesia. Patients with a pain intensity rating of ≥40 mm/100-mm by Visual Analog Scale following surgery received indomethacin submicron particle capsules (40 mg three times daily [TID] or twice daily [BID], or 20 mg TID), or placebo. One study included celecoxib (400 mg loading dose followed by 200 mg BID). The primary endpoint was the overall (summed) pain intensity difference measured by Visual Analog Scale over 48 h.
Results: Overall, 835 patients were enrolled in both studies with >350 patients in each study. In study 1, all doses of indomethacin submicron particle capsules provided significantly better analgesia than placebo (P ≤ 0.046). While celecoxib led to some pain control, it did not achieve statistical significance compared with placebo (P = 0.103; Table). In study 2, indomethacin submicron particle capsules 40 mg TID (P = 0.034) and BID (P = 0.023) provided significantly better analgesia than placebo (Table). In both studies, adverse events were generally similar across treatment groups and included nausea, localized post-procedural edema, dizziness, and headache.
Conclusion: Investigational, lower-dose indomethacin submicron particle capsules provided effective analgesia in two phase 3 studies in patients with acute pain following elective surgery. Indomethacin submicron particle capsules are a potentially promising lower-dose option for patients with acute pain.
Disclosure:
R. D. Altman,
Ferring Pharmaceuticals,
9,
McNeil Consumer & Specialty Pharmaceuticals,
5,
DePuy Synthes,
5,
Imprimis Pharmaceuticals, Inc,
5,
Oletec,
5;
A. Gibofsky,
GlaxoSmithKline plc,
1,
Bristol-Myers Squibb,
1,
Johnson & Johnson,
1,
Horizon Phartmaceuticals,
5,
Iroko Pharmaceuticals,
5,
Abbott Laboratories,
9,
Amgen, Inc,
9,
Genentech, Inc,
9;
M. Jaros,
Summit Analytical,
3;
C. Young,
Iroko Pharmaceuticals LLC,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lower-dose-indomethacin-submicron-particle-capsules-efficacy-in-acute-pain-results-from-two-phase-3-studies/