Session Information
Date: Tuesday, November 14, 2023
Title: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Treatment III: AxSpA
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Acute anterior uveitis (‘uveitis’), or ‘iritis’, is a common extra-musculoskeletal manifestation among patients (pts) with axial spondyloarthritis (axSpA).1 IL-17 has been implicated in the pathogenesis of uveitis; however, inhibition of IL-17A alone may not be optimal for the management of uveitis.2 Here, we report the incidence of uveitis following inhibition of IL-17A in addition to IL‑17F with bimekizumab (BKZ) in pts with axSpA.
Methods: Data were pooled for pts randomized to BKZ or placebo (PBO) in the double-blind treatment period (DBTP) of the phase (ph)3 studies BE MOBILE 1 (NCT03928704; non-radiographic [nr]-axSpA) and 2 (NCT03928743; radiographic [r]-axSpA i.e., ankylosing spondylitis).3,4 Data were pooled separately for all pts treated with BKZ 160mg every 4 weeks (Q4W) in BE MOBILE 1, BE MOBILE 2, BE MOVING (NCT04436640; open-label extension [OLE] of BE MOBILE 1 and 2), and the ph2b study BE AGILE (NCT02963506; r-axSpA)5 and its OLE BE AGILE 2 (NCT03355573). Uveitis treatment-emergent adverse events (TEAEs) were identified using the preferred terms “autoimmune uveitis”, “iridocyclitis”, “iritis”, and “uveitis”, and were reported as both incidence and exposure adjusted incidence rates (EAIRs) per 100 pt years (PY) for all pts who received ≥1 BKZ dose.
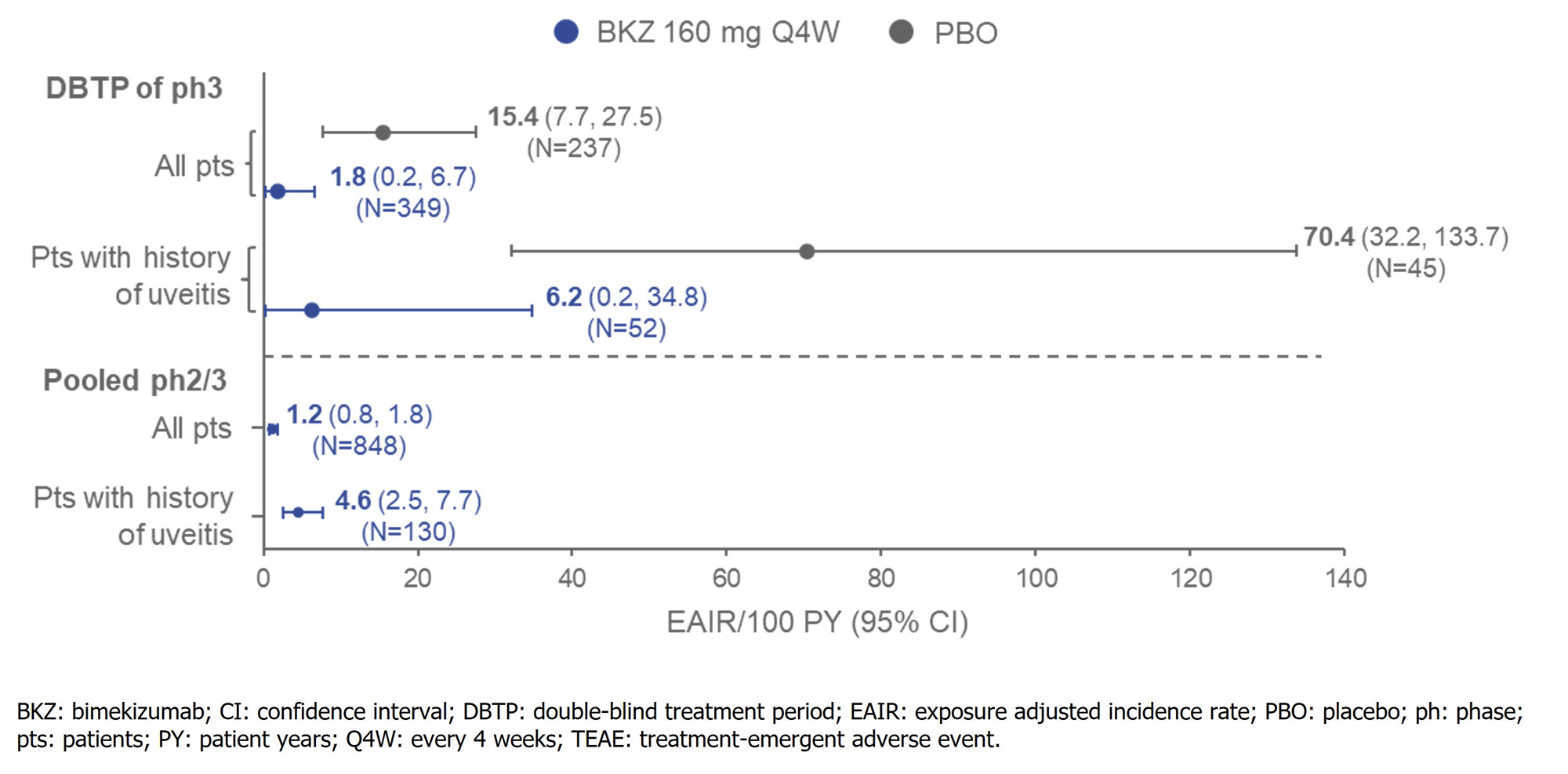
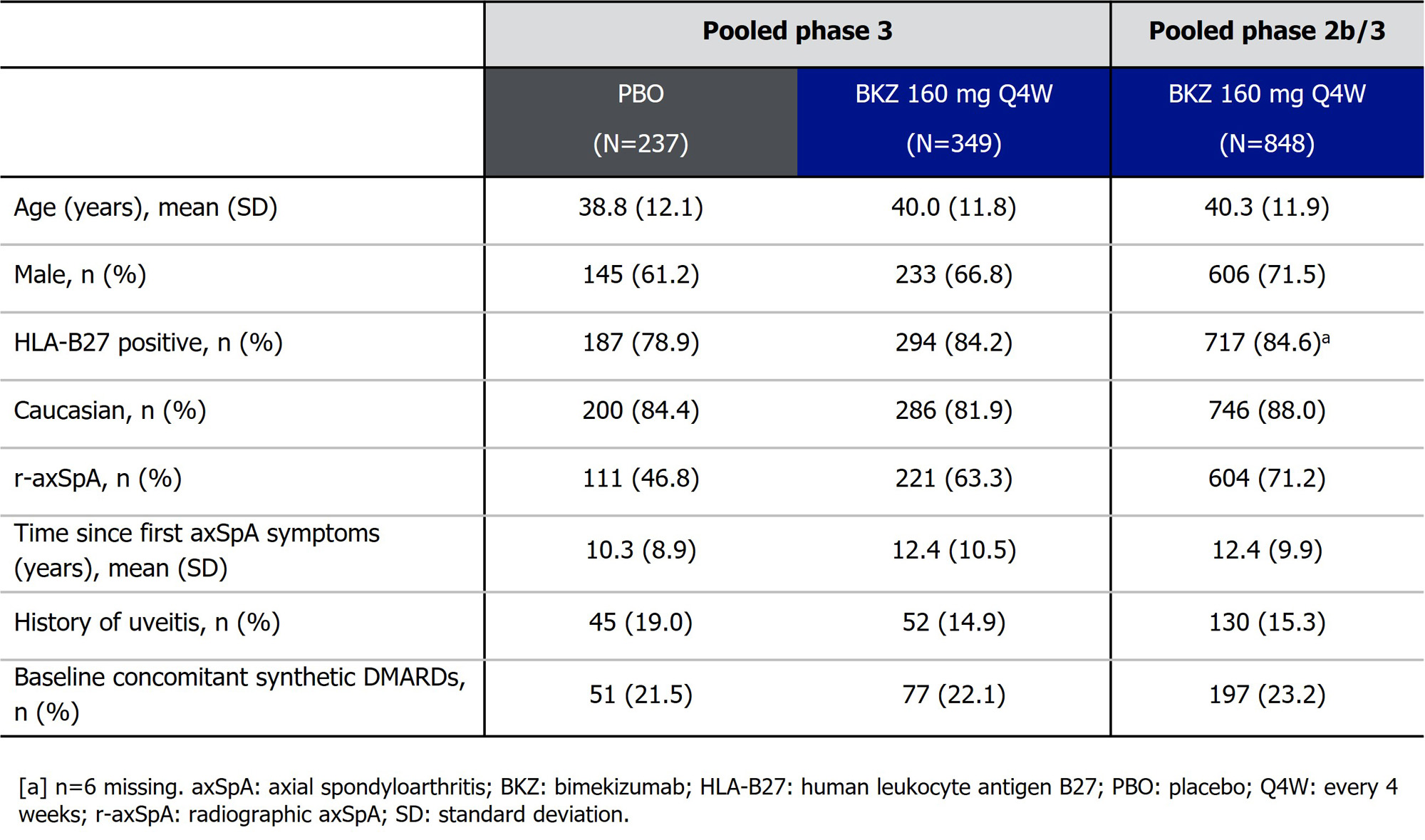
Results: Baseline characteristics were reflective of a pt population with moderate to severe axSpA (Table). In the DBTP of BE MOBILE 1 and 2, uveitis TEAEs occurred in 11/237 pts randomized to PBO (4.6%; EAIR/100 PY [95% CI]: 15.4 [7.7, 27.5]) and 2/349 (0.6%; 1.8 [0.2, 6.7]) pts randomized to BKZ (% difference [95% CI]: 4.07 [1.71, 7.60]); Figure). In the 45 PBO-randomized (19.0%) and 52 BKZ-randomized (14.9%) pts with history of uveitis, uveitis TEAEs occurred in 20.0% (EAIR/100 PY [95% CI]: 70.4 [32.2, 133.7]) and 1.9% (6.2 [0.2, 34.8]) of pts, respectively. In the pooled ph2b/3 trial data, total BKZ exposure was 2,034.4 PY (N=848); 130 (15.3%) pts had history of uveitis. Uveitis TEAEs occurred in 25 pts overall (2.9%; EAIR/100 PY [95% CI]: 1.2 [0.8, 1.8]) and 14 pts with history of uveitis (10.8%; 4.6 [2.5, 7.7]; Figure). All uveitis TEAEs were mild/moderate; one event led to discontinuation.
Conclusion: The incidence rate of uveitis TEAEs was lower to Wk 16 in axSpA pts randomized to BKZ 160 mg Q4W vs PBO. In the largest pool of ph2b/3 data available at the time of this report, the incidence rate of uveitis with BKZ 160 mg Q4W remained low at 1.2/100 PY.
References:1.Robinson PC. Arthritis Rheumatol. 2015;67(1):140–51; 2. Dick AD. J Opthalmol 2013;120(4):777–87; 3. Boel A. Ann Rheum Dis 2019;78:1545–9; 4. Baraliakos X. Arthritis Rheumatol 2022;74 (suppl 9); 5. van der Heijde D. Ann Rheum Dis 2020;79:595–604.
To cite this abstract in AMA style:
Rudwaleit M, Brown M, Van Gaalen F, Haroon N, Gensler L, Fleurinck C, Marten A, Massow U, De Peyrecave N, Vaux T, White K, Deodhar A, van der Horst-Bruinsma I. Low Uveitis Rates in Patients with Axial Spondyloarthritis Treated with Bimekizumab: Pooled Results from Phase 2b/3 Trials [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/low-uveitis-rates-in-patients-with-axial-spondyloarthritis-treated-with-bimekizumab-pooled-results-from-phase-2b-3-trials/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/low-uveitis-rates-in-patients-with-axial-spondyloarthritis-treated-with-bimekizumab-pooled-results-from-phase-2b-3-trials/