Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Acute anterior uveitis (‘uveitis’) is a common extra-musculoskeletal manifestation in patients (pts) with spondyloarthritis (SpA), and incidence varies with SpA type and disease duration.1,2,3 Interleukin (IL)-17 has been implicated in the pathogenesis of uveitis; however, previous data have not demonstrated the efficacy for IL-17A inhibition alone in managing the condition.4,5 The exposure-adjusted incidence rate (EAIR) per 100 pt-years (PY) of uveitis was lower in pts with axial SpA (axSpA) randomized to bimekizumab (BKZ; 1.8/100 PY), a dual IL-17A/F inhibitor, versus placebo (15.4/100 PY) after 16 weeks.6 Here, we report long-term incidence of uveitis following BKZ treatment in pts with axSpA or psoriatic arthritis (PsA).
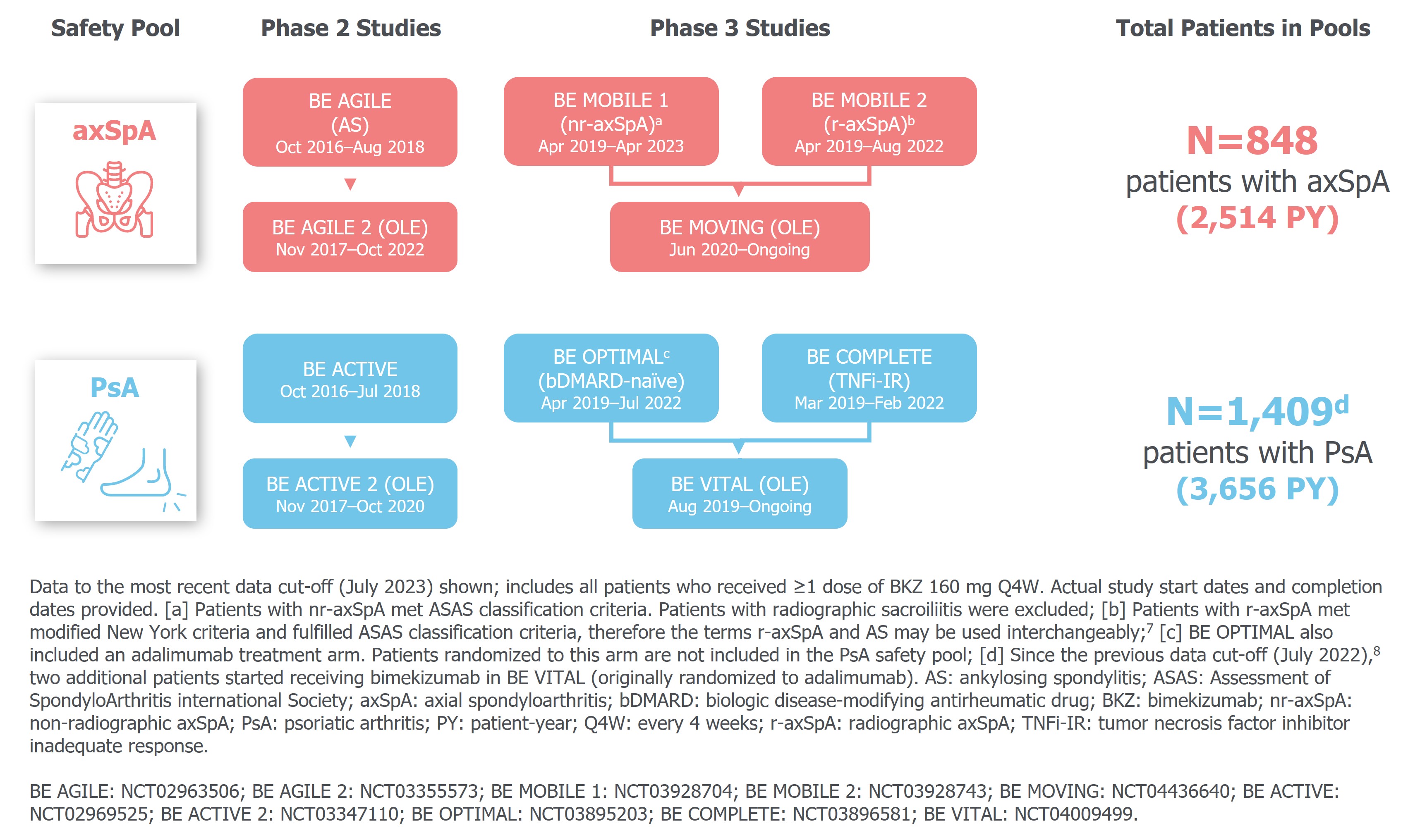
Methods: Safety data are reported for two pools, each comprising three phase 2b/3 (ph2b/3) studies and their open-label extensions, in pts with active axSpA (non-radiographic and radiographic axSpA) and active PsA, respectively (Figure 1).
Uveitis events were identified using the preferred terms “autoimmune uveitis”, “iridocyclitis”, “iritis”, and “uveitis”, coded according to MedDRA v19.0; note that “acute anterior uveitis” was not a specific preferred term available in MedDRA v19.0. Uveitis rates and EAIRs/100 PY for pts who received ≥1 BKZ 160 mg dose are reported (data cut-off: July 2023).
Results: Pts with axSpA (N=848) had a mean age (standard deviation [SD]) of 40.3 (11.9) years, and pts with PsA (N=1,409) had a mean age (SD) of 49.3 (12.4) years, with a mean time since diagnosis (SD) of 6.1 (7.8) and 7.0 (8.0) years, respectively. Of pts with axSpA, 130 (15.3%) had a history of uveitis; 21 (1.5%) pts with PsA had a history of uveitis. The majority of pts with axSpA were HLA-B27 positive (717/848 [84.6%]).
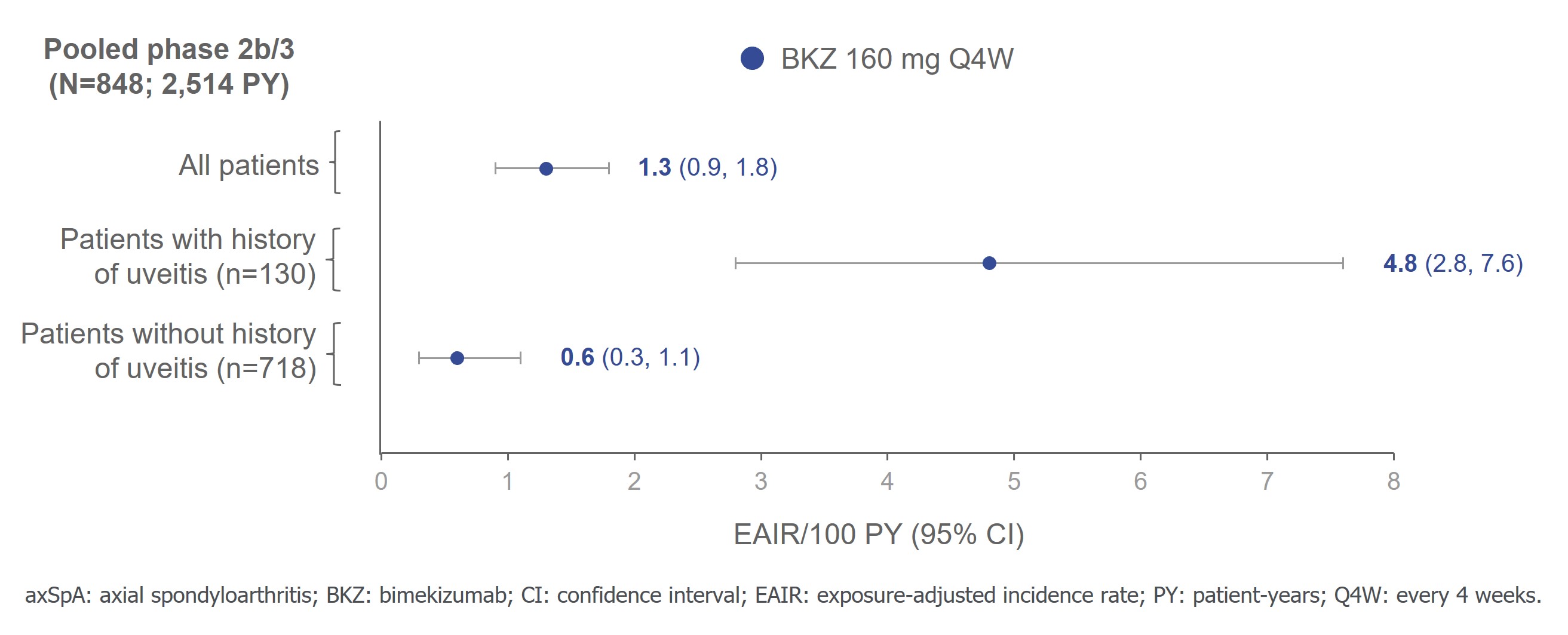
In pts with axSpA across the pooled ph2b/3 axSpA trial data, BKZ exposure was 2,514 PY. Uveitis occurred in 31/848 (3.7%; EAIR [95% CI]: 1.3/100 PY [0.9, 1.8]) pts overall and in 18/130 (13.8%; 4.8/100 PY [2.8, 7.6]) pts with history of uveitis. In pts without a history of uveitis, 13/718 (1.8%; 0.6/100 PY [0.3, 1.1]) pts had uveitis events (Figure 2). All events were mild/moderate, one led to treatment discontinuation.
Incidence of uveitis in pts with PsA was low across the pooled ph2b/3 PsA trial data (total BKZ exposure: 3,656 PY); uveitis occurred in three (0.2%; 0.1/100 PY [0.0, 0.2]) pts overall; one had a history of uveitis. No uveitis events led to treatment discontinuation.
Conclusion: Across 2,514 PY in pts with axSpA and 3,656 PY in pts with PsA, the long-term data suggest that the incidence of uveitis in pts treated with BKZ remains low.
References: 1. Robinson PC. Arthritis Rheumatol. 2015;67:140–51; 2. López-Medina C. RMD Open 2019;5:e001108; 3. Delmás A. RMD Open 2023;9:e002781; 4. Dick AD. J. Opthalmol. 2013;120:777–87; 5. Kwon OC. Rheumatol. 2024;keae003; 6. Rudwaleit M. Ann Rheum Dis. 2023;82:614–5; 7. Baraliakos X. Ann Rheum Dis. 2024;83:199–213. 8. Mease PJ. Arthritis Rheumatol. 2023;75(suppl 9). Abstract 0511.
To cite this abstract in AMA style:
van der Horst-Bruinsma I, Brown M, van Gaalen F, Haroon N, Gensler L, Marten A, Manente M, Stojan G, Vaux T, White K, Deodhar A, Rudwaleit M. Low Uveitis Rates in Patients with Axial Spondyloarthritis or Psoriatic Arthritis Treated with Bimekizumab: Long-Term Results from Phase 2b/3 Trials [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/low-uveitis-rates-in-patients-with-axial-spondyloarthritis-or-psoriatic-arthritis-treated-with-bimekizumab-long-term-results-from-phase-2b-3-trials/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/low-uveitis-rates-in-patients-with-axial-spondyloarthritis-or-psoriatic-arthritis-treated-with-bimekizumab-long-term-results-from-phase-2b-3-trials/