Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with systemic lupus erythematosus (SLE) are at an increased risk of developing osteoporosis and clinical fractures compared to healthy controls. Bone loss in SLE is multifactorial from the negative effects of systemic inflammation and use of glucocorticoids (GCs). The American College of Rheumatology recommends bone mineral density (BMD) testing within 6 months of starting chronic GC therapy for autoimmune conditions, including SLE. Prior studies have not investigated the frequency of BMD testing in the setting of chronic GC therapy and SLE using an electronic health record (EHR) cohort. Using a real-world, EHR cohort, we assessed the frequency of BMD testing in patients with SLE and GC therapy. We evaluated patient and provider factors impacting BMD testing.
Methods: We identified SLE cases from a de-identified EHR with over 2.9 million subjects with decades of follow-up using a previously validated and published algorithm. We chart reviewed to collect demographic, medication, and BMD data. We assessed for ever use of oral or intravenous GCs from inpatient and outpatient electronic prescribing systems, provider notes, and phone messages. Long term GC use was defined as ≥ 90 days of continuous use. Patients prescribed GCs for non-rheumatologic issues were excluded from review. The provider ordering BMD testing was recorded. Lowest T-scores were recorded from the femoral neck and lumbar spine. When multiple BMD tests were performed, the lowest T-score was recorded. Our primary outcome was frequency of BMD testing in SLE patients with long term GC therapy. We also evaluated for differences in SLE cases who had BMD testing vs. not using the Mann-Whitney U test for continuous variables and chi-square or Fisher’s exact test for categorical variables.
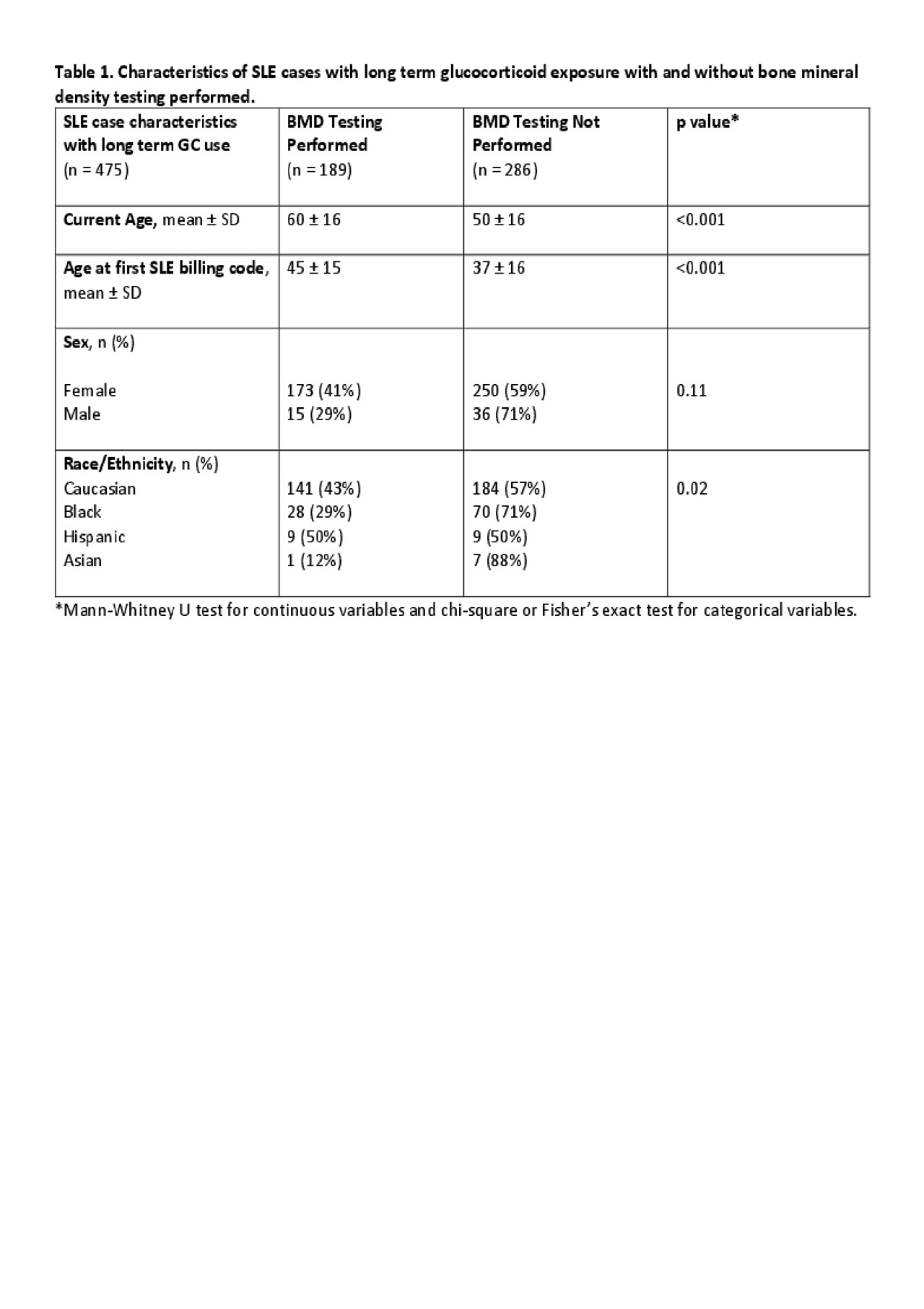
Results: We conducted chart review on 729 SLE cases. Of these cases, 539 had available medication duration data with 475 (88%) with long term GC use. SLE cases with long term GC use had a current mean age of 54 ± 17 years and were predominantly female (89%) with 72% Caucasian, 22% African American, 4% Hispanic and 2% Asian. The median age at first BMD testing was 51 years. BMD testing was ordered in 40% of cases with long term GC use. Rheumatologists accounted for ordering 75% of the BMD tests. The median time from start of GCs to first BMD testing was 2.5 years. The mean T-score of cases with long term GC use was -1.4 ± 0.9. There were no differences in sex between cases with or without BMD testing (Table 1). SLE cases with long term GC use and BMD testing were older compared to cases with long term GC use without BMD testing (60 ± 16 v. 50 ± 16, p < 0.001). Caucasians and Hispanics were more likely to have BMD testing than African Americans and Asians (p = 0.02).
Conclusion: Using a large EHR cohort, only 40% of SLE cases with long term GC use had BMD testing. SLE patients that were younger, African American, or Asian were all less likely to have BMD testing performed. These groups with the lowest rates of BMD testing are the same groups that are at highest risk of requiring a significant amount of GCs during their disease course and developing GC-related bone loss. Screening strategies for BMD testing should be implemented that target these high-risk groups.
To cite this abstract in AMA style:
Boone J, Tanner S, Barnado A. Low Rates of Bone Mineral Density Testing by Rheumatologists in Patients with Systemic Lupus Erythematosus and Glucocorticoid Therapy [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/low-rates-of-bone-mineral-density-testing-by-rheumatologists-in-patients-with-systemic-lupus-erythematosus-and-glucocorticoid-therapy/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/low-rates-of-bone-mineral-density-testing-by-rheumatologists-in-patients-with-systemic-lupus-erythematosus-and-glucocorticoid-therapy/