Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: In the pivotal fracture trial, FREEDOM, denosumab increased bone mineral density (BMD) and reduced the incidence of new vertebral, nonvertebral, and hip fractures in postmenopausal women with osteoporosis (Cummings SR et al, NEJM 2009). Denosumab reduced the risk of hip fracture in high-risk subgroups, including a 62% reduction in patients ≥75 years old (Boonen S et al, JCEM 2011). The effects of long-term denosumab treatment up to 10 years are being evaluated in the FREEDOM extension study. As fracture incidence increases with age, and in particular, hip fracture in women ≥75, we have further characterized the fracture incidence and BMD gains in women ≥75 who have been treated with denosumab for a total of 6 years.
Methods: During the extension, each woman has received 60 mg denosumab every 6 months and supplemental calcium and vitamin D daily. We evaluated the fracture incidence and BMD gains in women who completed 6 years of denosumab treatment (overall long-term group) and in the subset of these women who were ≥75 at FREEDOM baseline (higher-risk group).
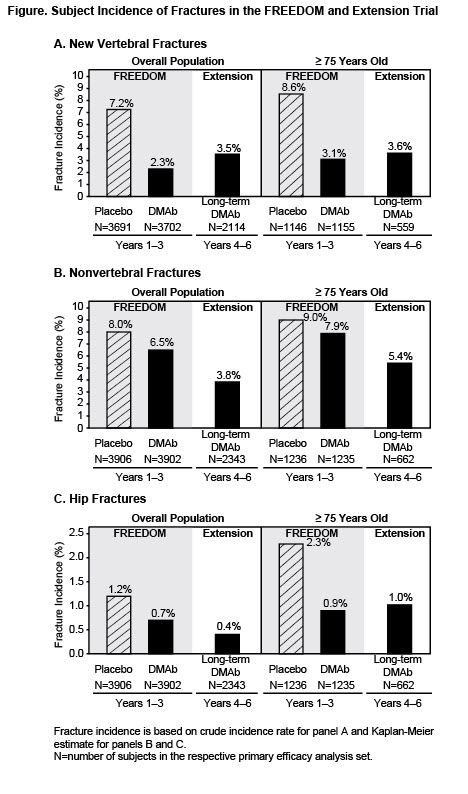
Results: The FREEDOM baseline characteristics for the overall long-term denosumab group (N=2343) and the higher-risk group (N=662) were similar except that those subjects in the higher-risk group were older (mean age: 72 years for overall and 78 years for higher-risk), and had a lower mean total hip BMD T-score (–1.9 for overall and –2.1 for higher-risk). Despite the increase in age of the subjects, denosumab treatment during years 4 to 6 continued to be associated with a low incidence of new vertebral, nonvertebral, and hip fractures. Furthermore, the incidence of fractures in the higher-risk group during years 4 to 6 was similar to what was originally observed in years 1 to 3 in women ≥75 treated with denosumab (Figure). BMD progressively increased over 6 years at the lumbar spine and total hip and was similar in women ≥75 compared with women in the overall long-term group. Despite advanced age, adverse events (AEs) and serious AEs in the higher-risk group in the extension were similar to the higher-risk group from FREEDOM, and these events did not increase over time with denosumab treatment.
Conclusion: Patients aged ≥75 are at higher risk of fracture than younger patients. Denosumab is a therapeutic option for the women ≥75 in whom the high risk of hip fracture is of particular concern. These results amplify the robust and consistent anti-fracture efficacy and safety profile of continued denosumab treatment over 6 years.
Disclosure:
S. Papapoulos,
Amgen Inc., Merck Co., Novartis, Eli Lilly, GSK,
5;
M. McClung,
Amgen Inc., Merck,
2,
Amgen Inc., Lilly, Merck, Novartis,
5,
Amgen Inc., Lilly, Novartis, Warner-Chilcott,
8;
N. Franchimont,
Amgen Inc., Biogenidec,
1,
Amgen Inc., Biogenidec,
3;
J. D. Adachi,
Amgen, Eli Lilly, GSK, Merck, Novartis, Procter & Gamble, Roche, Sanofi Aventis,
2,
Amgen, Eli Lilly, GSK, Merck, Novartis, Procter & Gamble, Roche, Sanofi Aventis, Warner Chilcott,
5,
Amgen, Eli Lilly, GSK, Merck, Novartis, Procter & Gamble, Roche, Sanofi Aventis, Warner Chilcott,
8;
H. G. Bone,
Amgen Inc.,
2,
Amgen Inc., Merck, Zelos, Tarsa, GSK,
5,
Amgen Inc. ,
8;
C. L. Benhamou,
Amgen Inc., Novartis, MSD, Servier, Roche, Lilly,
2,
Amgen Inc., MSD, Servier, Novartis ,
8;
J. Farrerons,
None;
J. Gallagher,
Amgen Inc.,
2;
J. Halse,
Amgen Inc.,
8;
K. Lippuner,
Amgen Inc., Eli Lilly ,
5;
S. Minisola,
Roche, GSK, Novartis, Nycomed, Sanofi-Aventis, Sigma Tau, Merck Sharpe, Amgen Inc., Chiesi Parmaceutica, ,
8,
Merck Sharp,
5;
O. Törring,
Amgen Inc., Nycomed Takeda,
5,
Amgen Inc., Nycomed Takeda,
8;
N. Daizadeh,
Amgen Inc.,
1,
Amgen Inc.,
3;
A. Wang,
Amgen Inc.,
3,
Amgen Inc.,
1;
R. B. Wagman,
Amgen Inc.,
1,
Amgen Inc.,
3;
S. Boonen,
Amgen Inc.,
2,
Amgen Inc.,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/low-fracture-incidence-is-maintained-in-postmenopausal-women-%e2%89%a575-years-with-osteoporosis-with-long-term-denosumab-treatment/