Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: B-lymphocyte stimulator was involved in the pathogenesis of SLE. The humanized monoclonal antibody belimumab with 10mg/kg was effective for active patients. The efficacy of low-dose belimumab for prevention of disease flares in patients with SLE with low disease activity was evaluated in this randomized trial (NCT04515719).
Methods: This was a 52-week, randomized, placebo-controlled trial. Patients who have Safety of Estrogens in Lupus Erythematosus National Assessment–Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) scores≤6; with no A score or no more than one B score on the British Isles Lupus Assessment Group (BILAG) scale; and who are treated with prednisone ≤20mg/day at screening were enrolled and randomly assigned in a 1:1 ratio to intravenous 120mg belimumab or placebo (saline) arm on weeks 0, 2, and 4, and then every 4 weeks until 48 weeks on the basis of standard of care (SOC). The primary outcome was a composite index of severe or mild-to-moderate disease flare (SELENA-SLEDAI Flare Index) within 52 weeks. Secondary outcomes included the percentage of severe flare, the percentage of mild-to-moderate flare, time to the first disease flare, changes in prednisone dose, SELENA-SLEDAI and safety analysis. The locked-down due to COVID-19 pandemic in Shanghai, China from April 2022 to June 2022 has greatly impacted patient recruitment and follow-up. Therefore, the trial was terminated prematurely at April 10th, 2022 when Renji Hospital, South campus was designated as a COVID-19 referral center during Shanghai locked-down.
Results: Overall, 90.5% of 116 patients receiving belimumab and 86.1% of 115 patients receiving placebo completed the study within a mean follow-up of 31.0 ± 16.1 weeks. Baseline characteristics were generally similar between treatment groups (Table 1). The primary endpoint was met. 7.8% (9/116) of patients receiving low-dose belimumab + SOC had disease flares within follow-up, which was significantly lower than that in patients receiving placebo + SOC, that is 19.1% (22/115) (p=0.012; difference 11.3%, OR 0.36 (95%CI 0.14~0.86); Figure 1A). Severe flares in belimumab group was only numerically lower than that in placebo group (1.7% vs 6.1%, p=0.1). Kaplan-Meier curves also demonstrated higher flare-free survival in patients receiving belimumab plus SOC (p=0.011, HR 2.63, 95% CI (1.21-5.72)) (Figure 1B). Glucocorticoid-sparing effects were observed in two groups (changes in prednisone from baseline to last visit, 1.64 ± 10.24 vs 0.91 ± 7.48, p=0.055). SELENA-SLEDAI was reduced by 0.62 ± 2.17 in belimumab group, however increased by 0.22 ± 2.82 in placebo group (p=0.004). Any adverse events were comparable between belimumab and placebo groups (61.2% vs 64.3%). Only 4 (3.4%) and 6 (5.2%) severe adverse events occurred in two groups (Table 2). There was no death.
Conclusion: Treatment with low-dose belimumab helped reduce disease flare risks in Chinese patients with SLE at low disease activity. Belimumab was generally well tolerated.
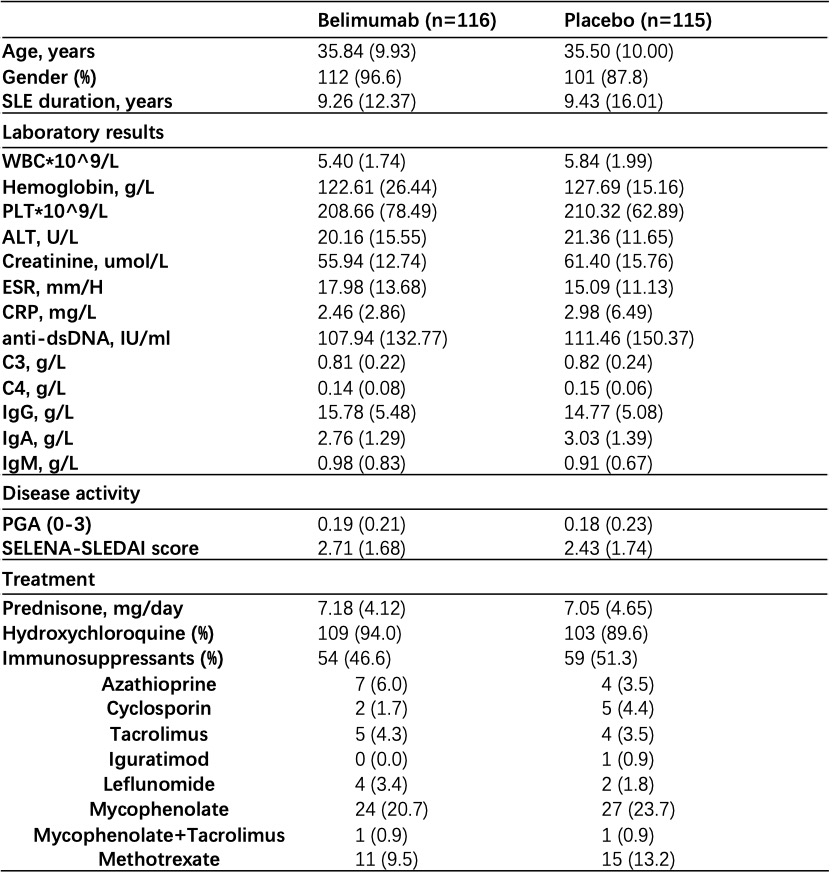 Table 1. Baseline characteristics of participants in two arms.
Table 1. Baseline characteristics of participants in two arms.
.jpg) Figure 1. The difference of overall disease flares between low-dose belimumab and placebo on the basis of SOC (A). Kaplan-Meier Curves demonstrated flare-free survival of participants from two arms (B).
Figure 1. The difference of overall disease flares between low-dose belimumab and placebo on the basis of SOC (A). Kaplan-Meier Curves demonstrated flare-free survival of participants from two arms (B).
To cite this abstract in AMA style:
Sun F, Wang H, Zhang D, Shen N, Chen S, Li T, Wan W, Dai S, Ye S. Low-dose belimumab reduced disease flares in patients with systemic lupus erythematosus at low disease activity: a multicenter, randomized, double-blind, placebo-controlled trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/low-dose-belimumab-reduced-disease-flares-in-patients-with-systemic-lupus-erythematosus-at-low-disease-activity-a-multicenter-randomized-double-blind-placebo-controlled-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/low-dose-belimumab-reduced-disease-flares-in-patients-with-systemic-lupus-erythematosus-at-low-disease-activity-a-multicenter-randomized-double-blind-placebo-controlled-trial/

.jpg)