Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: PsA-5Ts is a simple multidimensional composite measure, assessing pain, fatigue, physical function, skin problems, and depression, recently developed to measure overall health in PsA patients (pts) and correlate with established composite measures1. We assessed the longitudinal construct validity of the PsA-5Ts domains (PsA-5T-Ds) using data from three Phase 3 trials of the fully human IL-23p19-subunit inhibitor guselkumab (GUS), and evaluated the GUS effect on PsA-5T-Ds through week (W)24.
Methods: Pts in DISCOVER 1&2 (D1/D2; n=1120 ~90% bionaive) and COSMOS (n=285; inadequate response to 1 or 2 TNFi) had active PsA and were randomized to GUS 100 mg every 4 W (Q4W; D1/D2 only); GUS 100 mg at W0, W4, Q8W; or placebo (PBO). A PsA-5T-Ds score (range 0-100) was calculated1 based on the Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-F) scale, Health Assessment Questionnaire Disability Index (HAQ-DI), and question 28 (‘Have you felt downhearted and depressed’) of the 36-Item Short Form Survey (SF-36) to assess fatigue, physical function, and depression, respectively, after 0-10 transformation; pt pain and skin disease activity were assessed with native 0-10 visual analogue scales. Correlation of change in PsA-5T-Ds score with changes in PsA disease activity (DA) Score (PASDAS), DA Index for PsA (DAPSA), clinical (c) DAPSA, and SF-36 physical component summary score (PCS) was assessed with Pearson’s coefficient. Known-groups validity was assessed with mixed models for repeated measures (MMRM) using change in PsA-5T-Ds score as dependent variable; clinically meaningful improvements (CMI) in PASDAS (Δ≤-0.8), DAPSA (Δ≤-7.25), cDAPSA (Δ≤-5.7), and SF-36 PCS (Δ≥5) as independent variables; and age, sex, treatment, and BL PsA-5T-Ds score as covariates. GUS’ effect on PsA-5T-Ds score change and CMI achievement was assessed with multivariate MMRM and logistic regression, respectively.
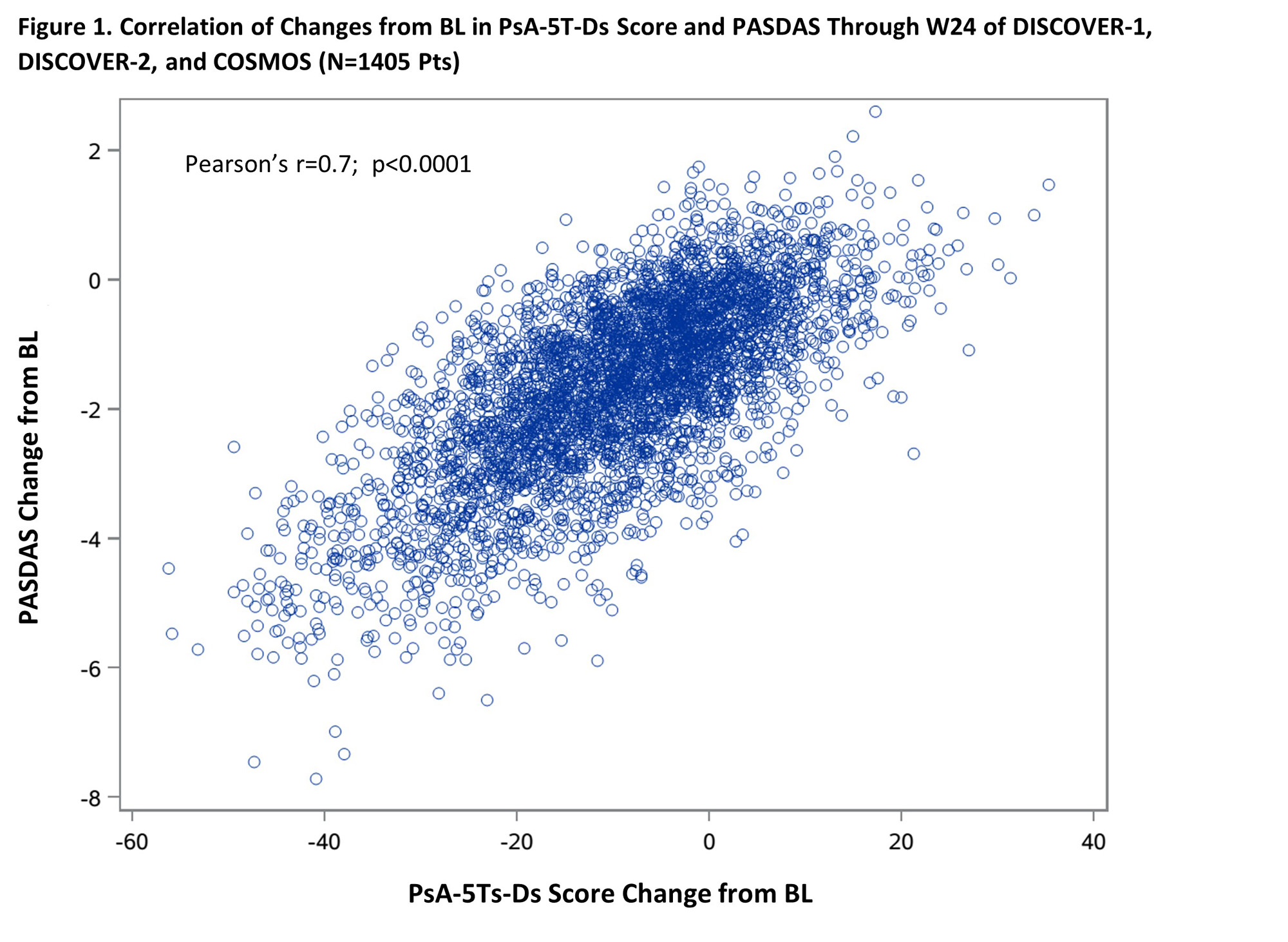
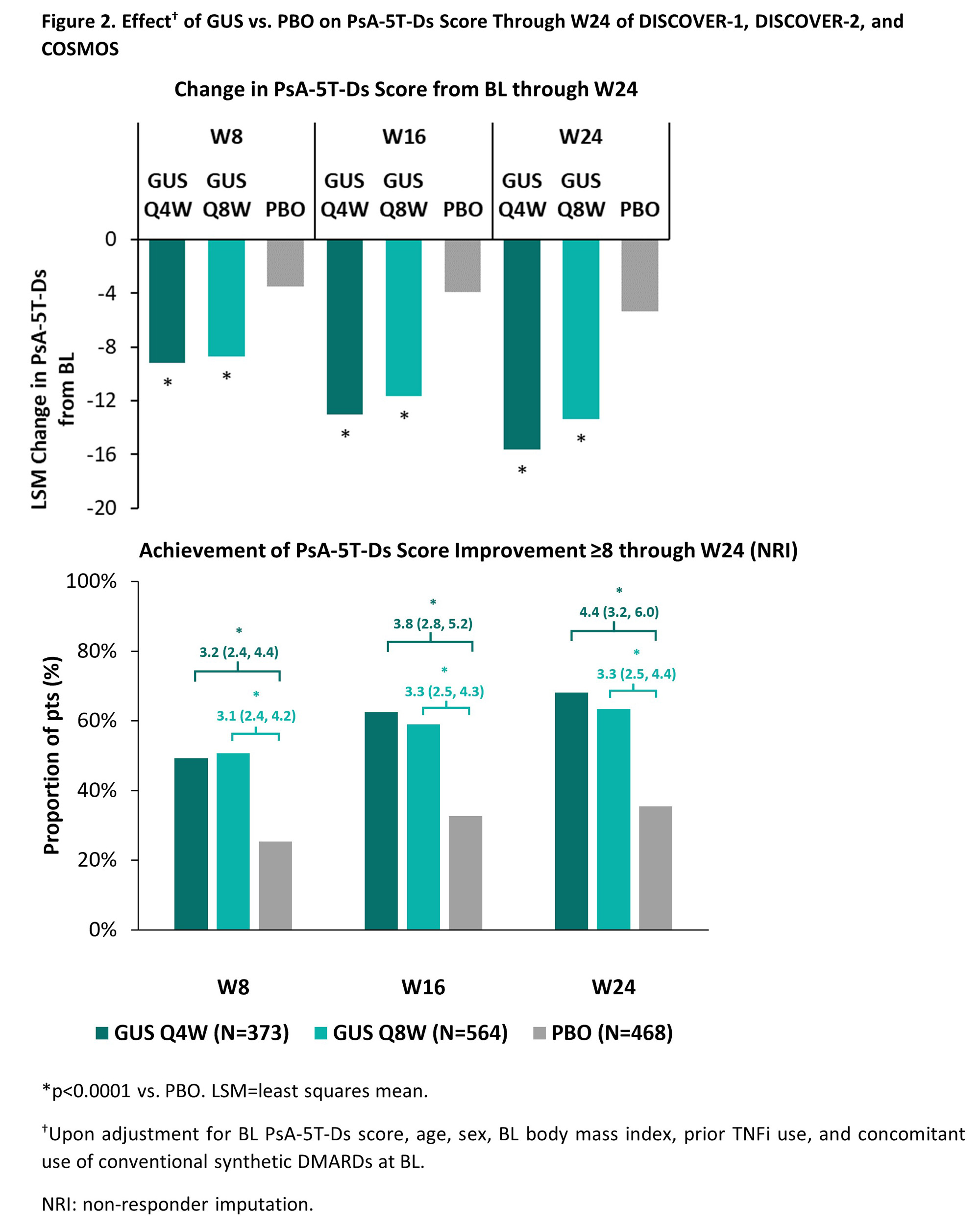
Results: Changes in PsA-5T-Ds score through W24 correlated strongly with variations in PASDAS (r=0.7; p< 0.0001) (Figure 1) and moderately with variations in DAPSA, cDAPSA, and SF-36 PCS (r=0.5; all p< 0.0001). CMI achievement in PASDAS, DAPSA, cDAPSA, and SF-36 PCS through W24 associated with significantly greater improvements in PsA-5T-Ds score vs non-achievement; corresponding time-weighted least square means (95% CI) differences of -7.9 (-8.5 to -7.3), -7.3 (-7.9 to -6.6), -7.2 (-7.9 to -6.6), and -5.9 (‑6.5 to -5.4), respectively, suggested a cutoff of ≥8 points for CMI in PsA-5T-Ds score. Upon adjusting for potential confounders, treatment with GUS Q4W or Q8W associated with significantly greater PsA-5T-Ds improvements through W24 and higher odds of achieving PsA-5T-Ds CMI vs PBO, starting at the first timepoint assessed (Figure 2).
Conclusion: In our first assessment of the PsA-5T-Ds longitudinal construct validity, we observed a strong correlation with PASDAS, which encompasses most core PsA domains, and good ability to discriminate between pts with and without CMI. GUS treatment was associated with greater improvements and higher rates of CMI achievement than PBO, supporting the established GUS efficacy across key PsA domains.
References
1. Salaffi F. Ann Rheum Dis 2022;81:218
To cite this abstract in AMA style:
Selmi C, Salaffi F, Aydin S, Soriano E, Rampakakis E, Sharaf M, Zimmermann M, Lavie F, Nash P, Mease P. Longitudinal Evaluation of the Novel Psoriatic Arthritis 5-Thermometer Scale (PsA-5Ts) Domains During Treatment with Guselkumab: Pooled Analysis of Three Phase 3, Randomized, Double-blind, Placebo-Controlled Studies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/longitudinal-evaluation-of-the-novel-psoriatic-arthritis-5-thermometer-scale-psa-5ts-domains-during-treatment-with-guselkumab-pooled-analysis-of-three-phase-3-randomized-double-blind-placebo-con/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-evaluation-of-the-novel-psoriatic-arthritis-5-thermometer-scale-psa-5ts-domains-during-treatment-with-guselkumab-pooled-analysis-of-three-phase-3-randomized-double-blind-placebo-con/