Session Information
Session Type: Abstract Session
Session Time: 2:15PM-2:30PM
Background/Purpose: Mass cytometry (CyTOF), a powerful tool to broadly assess immuno-phenotypes, has previously revealed that T follicular helper (Tfh) cells, T peripheral helper (Tph) cells, and age-associated B cells (ABCs) are robustly expanded in patients with newly diagnosed SLE. However, how these and other immune cell populations change over time in SLE remains unclear.
Methods: We employed CyTOF with two 39 marker panels (T and B cell) in cryopreserved peripheral blood mononuclear cells (PBMCs) from 9 patients with newly diagnosed immunosuppressant-naïve SLE, 15 patients with established SLE, and 14 non-inflammatory controls. FlowSOM (Flow Self-Organizing Maps) and marker analysis by tSNE (t-distributed Stochastic Neighbor Embedding) used to identify and quantify clusters based on their 39-parameter characterization. For the newly diagnosed cohort, PBMCs were analyzed at 3 time points (A = at diagnosis, B = 6 months after the diagnosis, C = 12 months after the diagnosis). Serum samples were also analyzed to quantify 65 cytokines by Luminex multiplex assay, and associations between cell types and cytokines assessed by Spearman correlation.
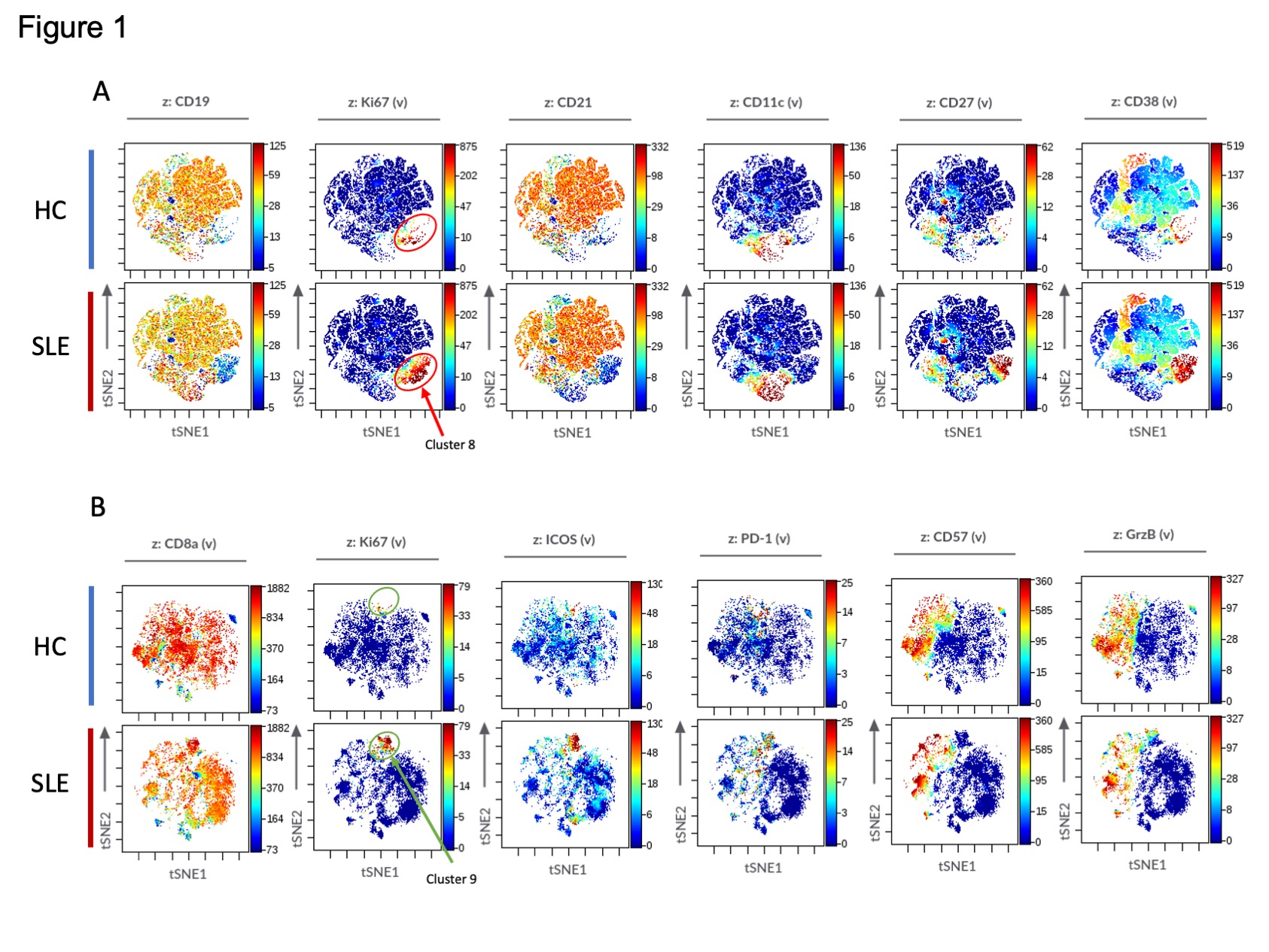
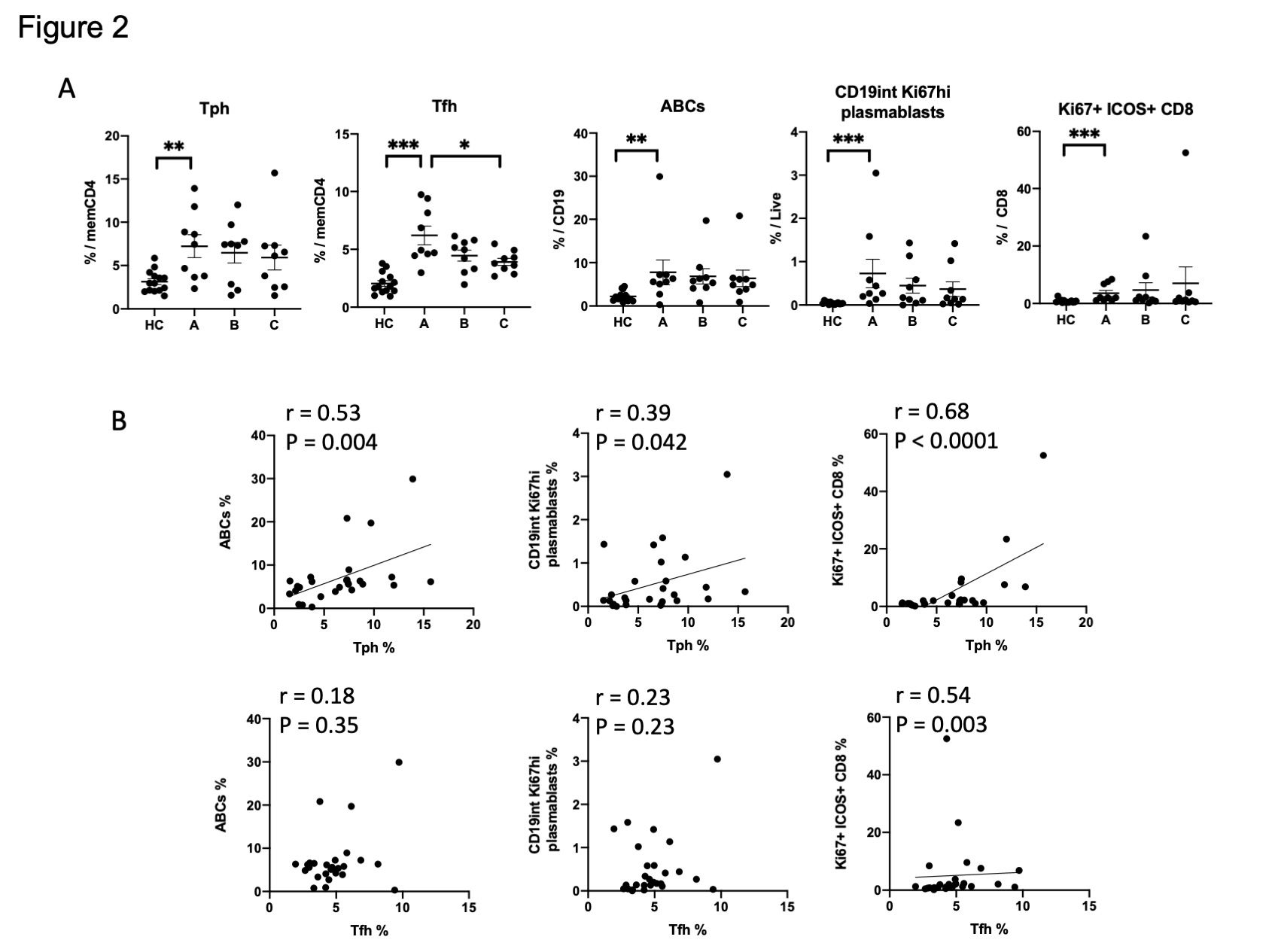
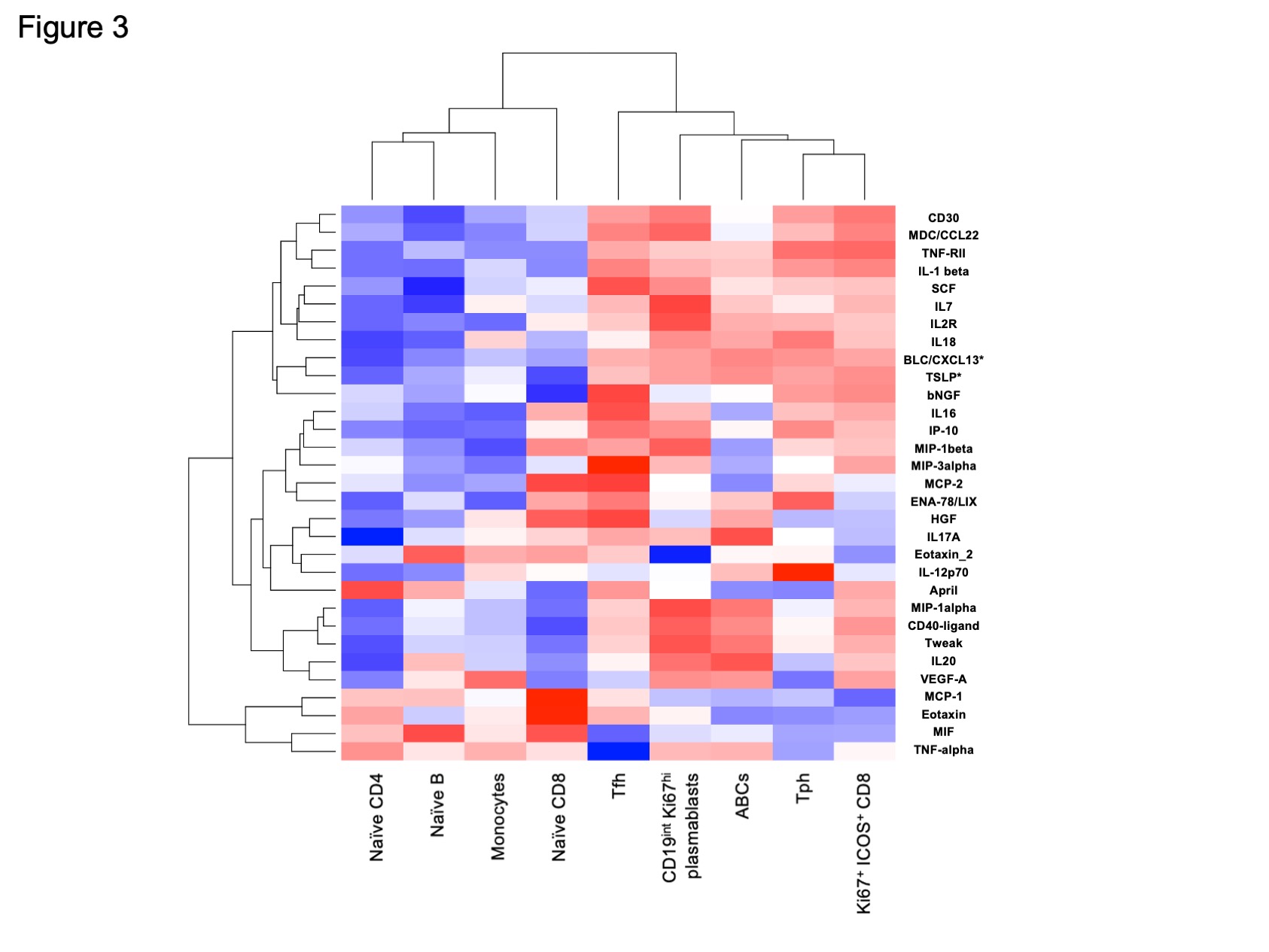
Results: We first confirmed that among CD4 T cells, Tfh cells (PD-1hi CXCR5+ CD4+ T cells) and Tph cells (PD-1hi CXCR5– CD4+ T cells) were significantly increased in SLE patients. A broad analysis of B cells identified 3 clusters as significantly increased in the patients with SLE. Two of these clusters contained CD11c+ CD21– ABCs, consistent with prior analyses. A third cluster, cluster 8, contained a CD19int Ki67hi B cell population. This CD19int Ki67hi cluster was also CD21low, CD11clow, CD27hi, and CD38hi, consistent with a Ki67hi plasmablast population (Figure1A). Among CD8 T cells, we identified one highly expanded cluster in SLE patients compared to controls, which expressed Ki67hi, ICOShi, PD-1int, CD57low, and granzyme Bint (Figure1B). In longitudinal analyses, the frequency of Tfh cells decreased over the first year of SLE, while Tph cells, ABCs, CD19int Ki67hi plasmablasts, and Ki67+ ICOS+ CD8 T cells remained elevated at 12 months (Figure 2A). Tph cells, but not Tfh cells, were correlated with ABCs (r = 0.53, p = 0.004) and CD19int Ki67hi PB (r = 0.39, p = 0.042). Ki67+ ICOS+ CD8 T cells were correlated with Tph cells and Tfh cells, but more strongly in Tph cells (r = 0.68, p < 0.0001) (Figure 2B). Correlation analyses including both immune cell frequencies and cytokines revealed an association of Tph cells, Ki67+ ICOS+ CD8 T cells, ABCs, and CD19int Ki67hi plasmablasts. These associated populations, but not Tfh cells, were also significantly correlated with CXCL13 and TSLP (Figure 3).
Conclusion: Tph cells, ABCs, and the two different Ki67+ proliferating populations were consistently elevated during the first year after diagnosis of SLE, while Tfh cells decreased in frequency over the same timeframe. This longitudinal immunophenotyping and cytokine profiling approach thus highlights persistent activation of a Tph-CXCL13-ABC- plasmablasts axis in both early and established phases of SLE.
To cite this abstract in AMA style:
Sasaki T, Bracero S, Chen L, Cao Y, Stevens E, Qu Y, Wang G, Keegan J, Lederer J, Alves S, Costenbader K, Rao D. Longitudinal CyTOF Immunophenotyping Reveals Distinct Patterns of T cell-B Cell Dysregulation in SLE [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/longitudinal-cytof-immunophenotyping-reveals-distinct-patterns-of-t-cell-b-cell-dysregulation-in-sle/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-cytof-immunophenotyping-reveals-distinct-patterns-of-t-cell-b-cell-dysregulation-in-sle/