Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Follow up of the ASAS Classification Cohort (CC) indicated a high positive predictive value for the ASAS classification criteria derived from baseline patient and imaging data1. Moreover, diagnosis of axSpA was changed by the local rheumatologist in only 11.2% of patients who were available at follow up at 4.4 years. This has raised potential concerns regarding diagnostic ascertainment bias. We aimed to determine the evolution of MRI features of axSpA from baseline to follow up by central reading of scans from this cohort, whether this reflects diagnostic assignment by the local rheumatologist, and the predictive utility of baseline MRI considered indicative of axSpA.
Methods: MRI images were available from 108 cases who had MRI performed at baseline and 4.4 years follow up and also had a local rheumatologist diagnosis at both time points. Eight experienced readers from the ASAS MRI group recorded MRI lesions in an eCRF that comprised global assessment (MRI indicative of axSpA: yes or no, active and/or structural lesion typical of axSpA present/absent according to ASAS definitions), whether the features met the criteria for an ASAS positive MRI, and detailed scoring of lesions per SIJ quadrant (SPARCC SIJ quadrantic method). MRI data from at least 2 readers and from the majority (≥5/8) was used to calculate positive and negative predictive values (PPV, NPV).
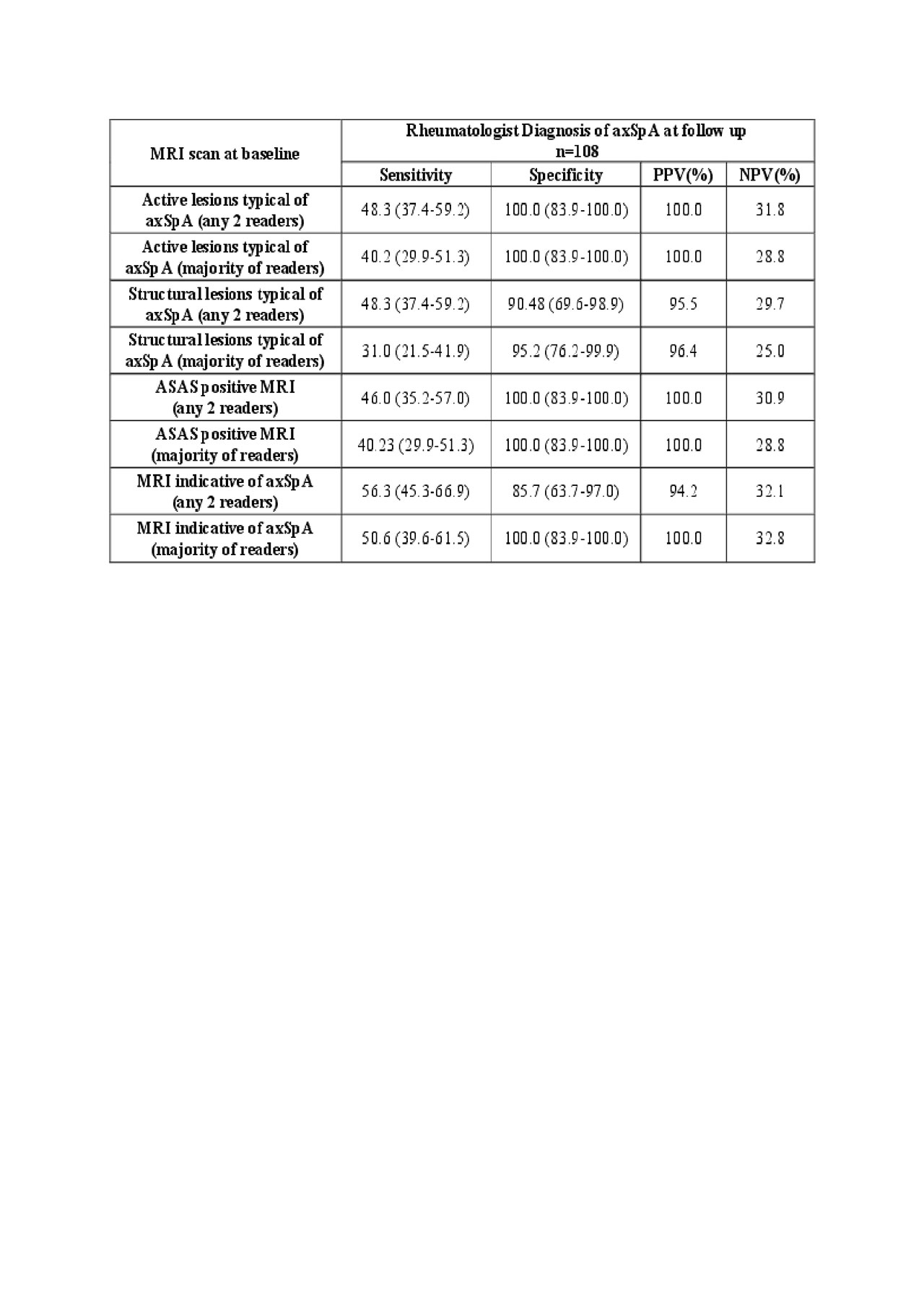
Results: MRI was considered indicative of axSpA in 52/108 (48.1%) at baseline and in 47/86 (54.7%) diagnosed as axSpA by the rheumatologist. Change in MRI diagnosis was recorded in only 10/108 (9.3%) of cases (4 from yes to no, and 6 from no to yes for axSpA) according to agreement by at least 2 readers (Table 1). Change in MRI diagnosis was recorded in only 3 cases according to a majority of readers. Change in rheumatologist diagnosis was recorded in 9/108 (8.3%), 2 of which had a change in MRI diagnosis. Baseline MRI had very high PPV for follow up diagnosis of axSpA (Table 2).
Conclusion: The lack of change in diagnostic ascertainment of rheumatologists over follow up of the ASAS-CC is supported by the evaluation of MRI scans by central reading. A positive MRI at baseline had very high PPV for a follow up diagnosis of axSpA.
1. Sepriano et al. ARD 2016;75:1034-42.
To cite this abstract in AMA style:
Maksymowych W, Baraliakos X, de Hooge M, Eshed I, Juhl Pedersen S, Weber U, Sieper J, Wichuk S, Poddubnyy D, Rudwaleit M, van der Heijde D, Landewé R, Paschke J, Lambert R, Østergaard M. Longitudinal Assessment of MRI of the Sacroiliac Joints in the ASAS Classification Cohort: Evolution of Diagnostic Features and Predictive Utility for Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/longitudinal-assessment-of-mri-of-the-sacroiliac-joints-in-the-asas-classification-cohort-evolution-of-diagnostic-features-and-predictive-utility-for-axial-spondyloarthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-assessment-of-mri-of-the-sacroiliac-joints-in-the-asas-classification-cohort-evolution-of-diagnostic-features-and-predictive-utility-for-axial-spondyloarthritis/