Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: There has been increased interest in understanding whether biologics can be safely withdrawn from patients (pts) receiving combination therapy with MTX once a clinical target has been achieved. The ability of the biologic to maintain the target as monotherapy following MTX withdrawal has received less consideration. The purpose of this analysis was to evaluate long-term clinical, functional, and radiographic outcomes in pts treated with open-label (OL) adalimumab (ADA) as monotherapy following attainment of low disease activity (LDA) with ADA+MTX.
Methods: PREMIER was a 2-year (yr), phase 3, randomized, controlled trial (RCT) in MTX-naïve pts with early RA who were randomized to MTX, ADA, or ADA+MTX.1 Pts completing the RCT were eligible to receive OL ADA for up to an additional 8 yrs (this trial is ongoing); MTX could be added at the investigator’s discretion. This post hoc analysis included data from pts treated with ADA+MTX during the RCT who reached at least an LDA state [defined as DAS28(CRP) <3.2] at Yr 2 (ie, the end of the RCT) and received OL ADA as monotherapy up to Yr 5. The percentages of pts remaining in LDA or with normal function (HAQ-DI <0.5) at Yr 5 were summarized using non-responder imputation based on the population entering the OL period and as observed for pts with data available at Yr 5. Mean ΔmTSS and the proportion without radiographic progression (ΔmTSS ≤0.5) from Yrs 2-5 were summarized as observed. Conditional logistic regression analysis based on propensity score matching was used to identify variables significantly associated with MTX use.
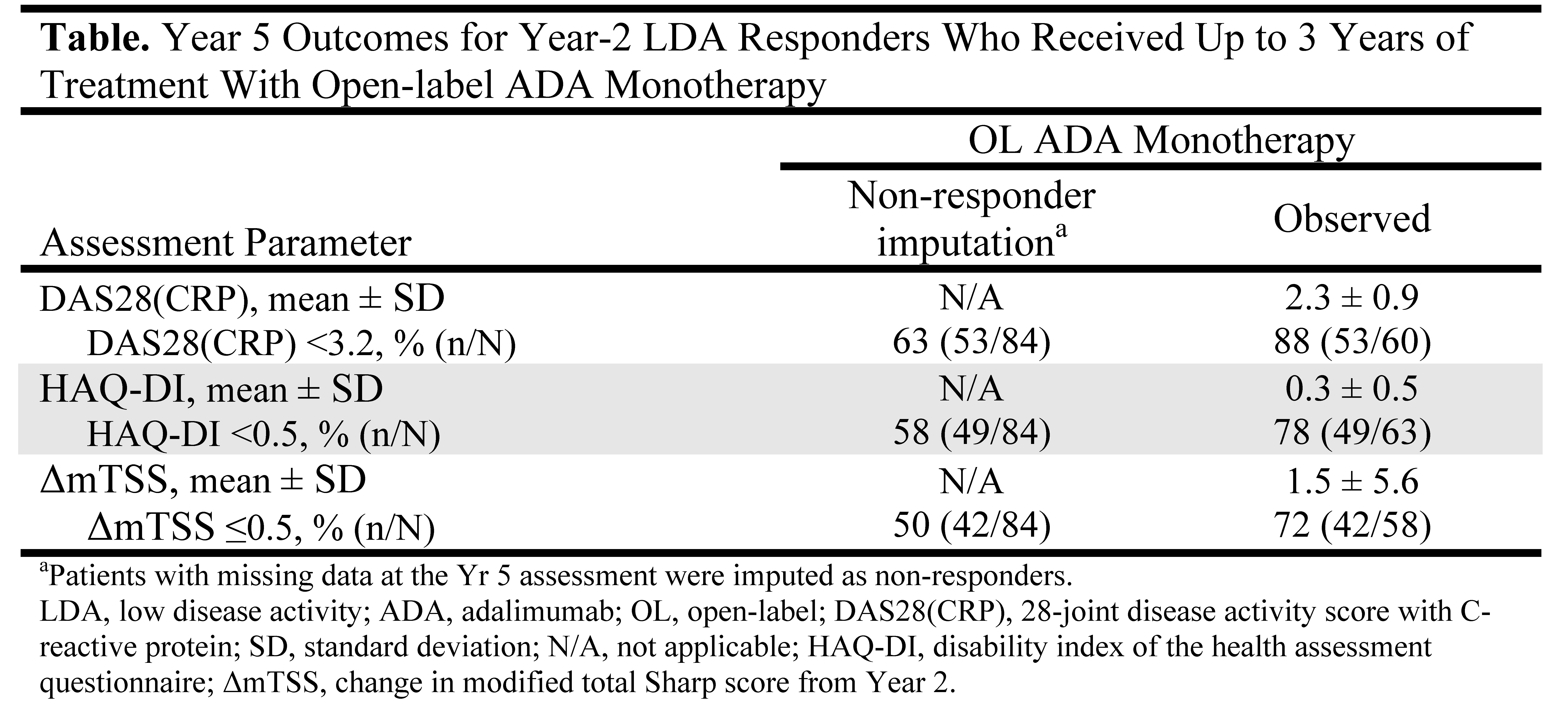
Results: Of the 183 ADA+MTX-treated pts who enrolled in the OL extension, 140 (83%) reached an LDA state at Yr 2. Among the LDA responders, 84 (60%) received ADA as monotherapy during the OL period, and 56 (40%) reinitiated MTX during the OL extension (time to 1st MTX use: mean/median=28/5 weeks). Higher physician’s global assessment appeared to predict MTX use during the OL extension (P <.01). A total of 60 pts (75%) completed 3 yrs of OL ADA monotherapy. Adverse events were the most frequently cited reason for study discontinuation (n=9); no pt withdrew citing loss of efficacy. The majority of pts retained LDA at Yr 5 with ADA monotherapy (63%; Table); 50% of the 84 pts were in DAS28(CRP) remission and 58% had normal function at Yr 5. Of the pts with Yr 5 data available, 88% were in LDA. Further, OL ADA monotherapy was associated with clinically insignificant radiographic progression for pts completing Yr 5 (mean annual mTSS progression rate from Yrs 2-5 =0.5 units/yr).
Conclusion: Following attainment of an LDA state at Yr 2 with ADA+MTX combination therapy, treatment with OL ADA as monotherapy for 3 yrs was sufficient for most pts to retain LDA and have minimal radiographic progression. Thus, MTX withdrawal appears possible for some pts whose disease activity is responsive to ADA monotherapy.
Reference: 1Arthritis Rheum 2006;54:26–37.
Disclosure:
E. Keystone,
Abbott, Amgen, AstraZeneca, BMS, Centocor, Genzyme, Merck, Novartis, Pfizer, Roche, and UCB,
2,
Abbott, AstraZeneca, Biotest, BMS, Centocor, Genentech, Merck, Nycomed, Pfizer, Roche, and UCB,
5,
Abbott, Amgen, BMS, Janssen, Merck, Pfizer, Roche, and UCB,
8;
F. C. Breedveld,
Centocor, Schering-Plough, Amgen/Wyeth, and Abbott,
5;
H. Kupper,
Abbott Laboratories,
1,
Abbott Laboratories,
3;
S. Liu,
Abbott Laboratories,
1,
Abbott Laboratories,
3;
S. Florentinus,
Abbott Laboratories,
1,
Abbott Laboratories,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-use-of-adalimumab-as-monotherapy-following-attainment-of-low-disease-activity-with-adalimumab-plus-methotrexate/