Session Information
Date: Tuesday, November 10, 2015
Title: Spondylarthropathies and Psoriatic Arthritis - Clinical Aspects and Treatment Poster III: Therapy
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The tolerability and
efficacy of golimumab (GLM) as a treatment for nonradiographic axial
spondyloarthritis (nr-axSpA) were recently investigated in a randomized,
double-blind (DB), placebo (PBO)-controlled, phase 3 study (GO-AHEAD).1
We report the findings from an open-label extension (OLE) of GO-AHEAD that evaluated
the long-term use of GLM in patients with nr-axSpA.
Methods: Patients completing
the 16-week DB study were eligible to receive open-label GLM 50 mg every 4
weeks during the 44-week extension (36-week treatment period; 8-week safety
follow-up). Safety evaluations included the incidence/severity of adverse
events (AEs). Efficacy evaluations included ASAS 20, ASAS 40, BASDAI 50, ASAS
partial remission (PR; ≤20 mm score in all 4 domains), and ASDAS-C at
weeks 20, 24, 32, 40, and 52. Quality of life evaluations included EQ-5D and
percentage of work impairment (WPAI) at weeks 16 and 52. Data were summarized
descriptively; all patients were included. Non-responder imputation was used
for missing ASAS 20, ASAS 40, and ASAS PR values. BASDAI required 3 of 5
responses; LOCF imputation was used for missing values. ASDAS-C was only calculated
if all components were available.
Results: Of the
197 subjects treated in the DB study, 189 entered the OLE (GLM/GLM, 93/97
[96%]; PBO/GLM, 96/100 [96%]). In total, 174/189 (92%) patients completed all
visits (GLM/GLM, 85/93 [91%]; PBO/GLM, 89/96 [93%]). There were no
notable differences in the number/types of AEs between the GLM/GLM and PBO/GLM
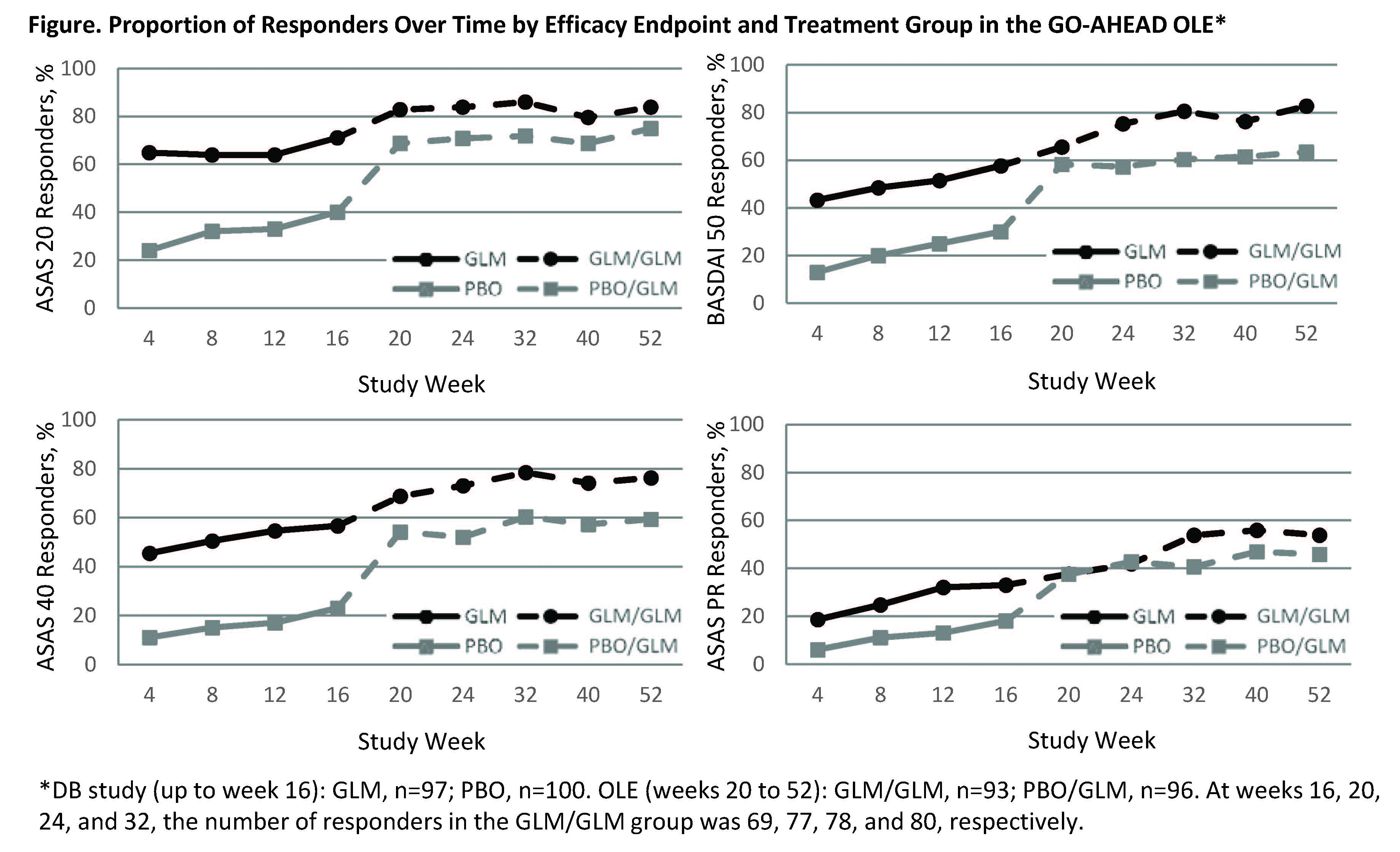
groups (Table). For ASAS 20, ASAS 40, BASDAI 50, and ASAS PR, the PBO/GLM group
showed notable improvement in these measures after switching to GLM in the OLE,
while the proportions of responders in the GLM/GLM group remained higher than
the PBO/GLM group throughout the study (Figure). For week 52 vs week 16, mean
changes from baseline (BL) in ASDAS-C were similar or better in the GLM/GLM
group (–2.2 vs –1.7) and were improved in the PBO/GLM group after switching to
GLM (–1.7 vs –0.6). At week 52, the mean change from BL in EQ-5D Health State
VAS score was 3.2 cm in the GLM/GLM group and 2.3 cm in the PBO/GLM group, and
the mean change from BL in the percentage of work impairment while working was
–28.1% in the GLM/GLM group and –22.8% in the PBO/GLM group.
|
Table. Summary of Adverse Events in the GO-AHEAD OLE |
|||
|
AEs,a n (%) |
GLM/GLM N=93 |
PBO/GLM N=96 |
Total N=189 |
|
Treatment-emergent AE |
39 (41.9) |
52 (54.2) |
91 (48.1) |
|
Treatment-related AEb |
12 (12.9) |
16 (16.7) |
28 (14.8) |
|
Nasopharyngitis |
2 (2.2) |
3 (3.1) |
5 (2.6) |
|
Upper respiratory tract infection |
2 (2.2) |
2 (2.1) |
4 (2.1) |
|
Headache |
2 (2.2) |
2 (2.1) |
4 (2.1) |
|
Discontinuation due to AEc |
1 (1.1) |
2 (2.1) |
3 (1.6) |
|
Serious AEd |
2 (2.2) |
3 (3.1) |
5 (2.6) |
|
Fatal AE |
0 |
0 |
0 |
|
aIncludes patients who received ≥1 dose of study drug. bThe most common AEs considered treatment-related (occurring in ≥3 pts in total) are shown. cGLM/GLM: acute tonsillitis and treatment-related bacterial infection (n=1); PBO/GLM: hepatitis, treatment-related rhinitis (each n=1). dGLM/GLM: bacterial infection, duodenitis (each n=1); PBO/GLM: treatment-related migraine, uterine polyp, staphylococcal infection (each n=1). |
|||
Conclusion: Consistent with results of the
GO-AHEAD randomized trial,1 treatment with GLM in the OLE was
generally well tolerated in patients with nr-axSpA. Improvements in disease activity were retained in
patients who received GLM in the OLE and in patients who switched from PBO to
GLM.
Reference
1. Sieper J,
et al. Arthritis
Rheum.
2014;66:S1283-S1284.
To cite this abstract in AMA style:
van der Heijde D, Dougados M, Maksymowych W, Bergman G, Curtis SP, Tzontcheva A, Philip G, Huyck S, Sieper J. Long-Term Tolerability and Efficacy of Golimumab in Active Nonradiographic Axial Spondyloarthritis: Results from the Open-Label Extension of a Randomized, Double-Blind Study [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/long-term-tolerability-and-efficacy-of-golimumab-in-active-nonradiographic-axial-spondyloarthritis-results-from-the-open-label-extension-of-a-randomized-double-blind-study/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-tolerability-and-efficacy-of-golimumab-in-active-nonradiographic-axial-spondyloarthritis-results-from-the-open-label-extension-of-a-randomized-double-blind-study/