Session Information
Date: Sunday, November 13, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Tapering of TNF blockers (TNFb) in rheumatoid arthritis (RA) patients in sustained remission is feasible in short-term randomized controlled trials (RCT). Less data are available on the sustainability of such a strategy over a longer term in real life settings.
Methods: The STRASS investigators of the STRASS trial were contacted between April and October 2015, i.e., 3 years after the end of the trial, to collect evolution data at 1, 2 and 3 years after trial completion of patients having completed the RCT. During this long-term follow-up period, physicians were free to either keep the same regimen as in the arm patients were initially randomized or change injections frequency or drugs. The main endpoints were the rate of patients with spaced TNFb injections, the rate of TNFb-free patients and the mean TNFb dose compared to full dose or dose quotient (as a % of full dose).
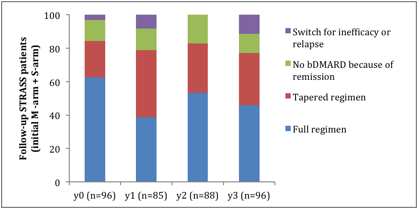
Results: Ninety-six patients (76.2% of the completer population) had data available up to 3 years. At this term, mean DAS28 was 2.6±1.3 (respectively 2.7±1.4 in the initial Maintenance-arm, 2.6±1.2 in the initial Spacing-arm, p=0.89) and 72.9% were in low disease activity or remission (respectively 72.1% and 73.0%, p=0.93) (Table 1). Thirty patients (31.2%) had a tapered regimen (respectively 30.8% and 31.8%, p=0.96) and 11.5% (respectively 5.8% and 18.2%, p=0.07) discontinued TNFb (Figure 1). The mean TNFb dose quotient among the 96 patients was 72% of full dose (respectively, 77 and 66%, p=0.16). Eighteen (30.2%) had structural damage progression during the follow-up (respectively 25.0% and 36.8%).
Conclusion : Sustained TNFb de-escalation (injection tapering or discontinuation) is achievable in only 43% of patients over 3 years with limited dose reduction (28% on average). More optimal strategies remain needed to durably maintain remission in patients for whom bDMARD have been tapered or discontinued. Table 1 Patient outcome over 3 years of follow up
|
Baseline
|
3-year follow-up |
|||
| Total (n=96) | Total (n=96) | Initial M-arm (n=52) | Initial S-arm (n=44) | |
| Disease activity |
|
|
|
|
| Tender joint count (28 joints) | 2.4±4.9 | 1.5±2.5 | 1.8±2.9 | 1.1±1.9 |
| Swollen joint count (28 joints) | 1.0±1.9 | 1.1±2.5 | 1.2±2.8 | 0.9±2.1 |
| ESR, mm/1st hour | 15.9±11.7 | 17.8±15.7 | 18.8±2.8 | 16.6±10 |
| CRP, mg/L | 3.8±5.2 | 5.6±12.5 | 7.4±16,2 | 3.6±4.6 |
| Normal acute phase reactant , n (%) | 59 (61.5) | 54 (56.2) | 30 (57.7) | 24 (54.5) |
| DAS28 | 2.4±1.2 | 2.6±1.3 | 2.7±16,2 | 2.63±1.2 |
| bDMARD intake | ||||
| Any TNFb | 82 (85.4) | 72 (75.0) | 41 (78.8) | 31 (70.4) |
| ADA, n (%) | 35 (36.5) | 30 (31.2) | 17 (32.7) | 13 (29.5) |
| ETA, n (%) | 47 (48.9) | 42 (43.7) | 24 (46.1) | 18 (40.9) |
| Other bDMARD, n (%) | 2 (2.1) | 13 (13.5) | 8 (15.4) | 5 (11.4) |
| Full dose, n (%) | 60 (62.5) | 44 (45.8) | 27 (51.9) | 17 (38.6) |
| Tapered regimen, n (%) | 21 (21.9) | 30 (31.2) | 16 (30.8) | 14 (21.8) |
| Discontinuation, n (%) | 12 (12.5) | 11 (11.5) | 3 (5.8) | 8 (18.2) |
| Switch from another bDMARD | 4 (4.2) | 18 (18.7) | 10 (19.2) | 8 (18.2) |
| Dose quotient, mean (sd) | 0.75±0.37 | 0.72±0.36 | 0.77±0.33 | 0.66±0.39 |
To cite this abstract in AMA style:
Sigaux J, Bailly F, Gandjbakhch F, Foltz V, Tubach F, Gossec L, Fautrel B. Long-Term Sustainability of TNF-Blocker Injection Spacing in Rheumatoid Arthritis: Results of a 3-Year Long-Term Observational Follow-up of a Tapering randomised Controlled Trial [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/long-term-sustainability-of-tnf-blocker-injection-spacing-in-rheumatoid-arthritis-results-of-a-3-year-long-term-observational-follow-up-of-a-tapering-randomised-controlled-trial/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-sustainability-of-tnf-blocker-injection-spacing-in-rheumatoid-arthritis-results-of-a-3-year-long-term-observational-follow-up-of-a-tapering-randomised-controlled-trial/