Session Information
Date: Monday, October 27, 2025
Title: (1434–1466) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Tildrakizumab (TIL) is an anti–IL-23 p19 monoclonal antibody approved for the treatment of adults with moderate-to-severe plaque psoriasis. In a Phase 2b trial in patients with active psoriatic arthritis (PsA; NCT02980692), TIL treatment significantly improved joint and skin symptoms and was well tolerated for up to 52 weeks. An open-label long-term extension (LTE) of that trial evaluated the safety of TIL through Week (W)208 of treatment in patients with active PsA.
Methods: Patients who completed treatment in the Phase 2b parent study, achieved ACR20 response at W52, and had sufficient clinical benefit per the investigator were eligible for the LTE. Patients continued to receive the same dose of TIL as in the parent study (200 mg every 4 weeks, 200 mg every 12 weeks [Q12W], or 100 mg Q12W) until parent study database lock (at least 52 weeks of treatment), after which all patients received TIL 100 mg Q12W through W208. Safety was assessed in all patients who entered the LTE and received ≥1 dose of TIL based on frequency and severity of adverse events (AEs); frequency of serious AEs (SAEs), AEs of special interest (AESIs), and AEs of clinical interest (AECIs); and presence of antidrug antibodies (ADAs) to TIL. Analyses were based on the treatment received.
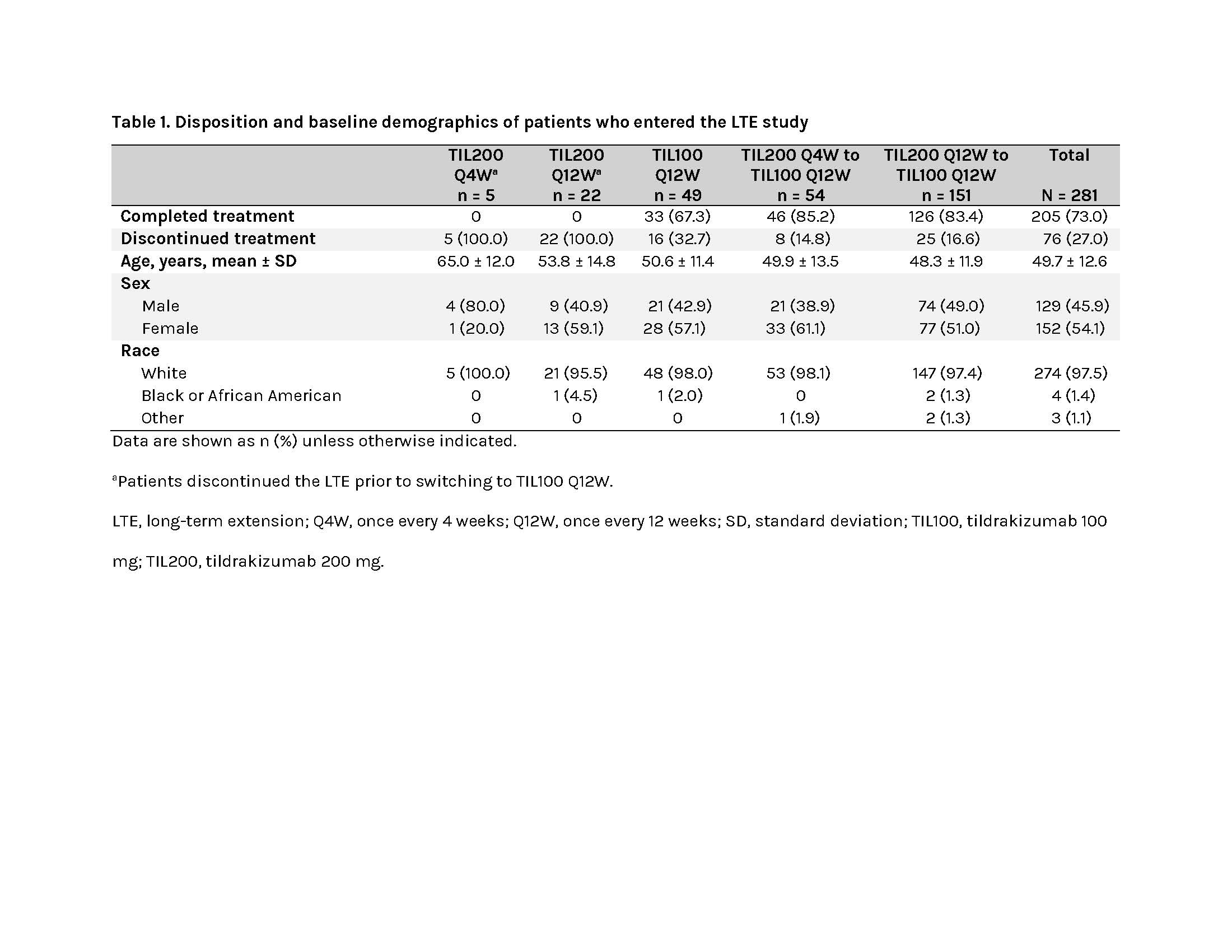
Results: Of 303 patients from the parent study screened, 281 entered the LTE and received ≥1 dose of TIL, and 205 completed treatment through W208 (Table 1).Any AEs, treatment-related AEs, SAEs, and treatment-related SAEs occurred in 79.7% (224/281), 19.6% (55/281), 14.2% (40/281), and 0.7% (2/281) of patients, respectively (Table 2). The most common treatment-related AEs were upper respiratory tract infection (3.2%) and nasopharyngitis (2.5%). A total of 6.0% (17/281) and 2.5% (7/281) of patients discontinued the study due to AEs and treatment-related AEs, respectively (Table 2), most due to infections and infestations. AESIs were reported in 4.6% (13/281) of patients and included infections and infestations (1.8%); cardiac disorders (1.1%; included 2 acute myocardial infarction events confirmed as major adverse cardiovascular events, not related to the study drug); malignancies (n = 2; 0.7%); respiratory, thoracic, and mediastinal disorders (n = 2; 0.7%); and vascular disorders (n = 1; 0.4%; Table 2). Only 1 AECI (viral infection) occurred during the LTE. AEs leading to death were reported in 2 patients (1 aortic dissection and 1 metastatic adenocarcinoma), both deemed unrelated to the study drug (Table 2).A total of 2.8% (8/281) and 1.1% (3/281) of patients were positive for non–treatment-emergent (TE) and TE ADAs, respectively; 0.7% (2/281) and 0.4% (1/281) had non–TE and TE neutralizing antibodies (nAbs; Table 3). Patients’ TE ADA-positive or nAb-positive statuses were not associated with AEs of hypersensitivity or injection-site reactions, AESIs, or AECIs. The effects of ADAs and nAbs on TIL clearance could not be quantified due to the low number of ADA-positive patients.
Conclusion: The safety of TIL was maintained through W208 of treatment in patients with PsA, with no new safety signals. These data support continued development of TIL to treat patients with active PsA.
To cite this abstract in AMA style:
Mease P, Chohan S, Garcia Fructuoso F, Luggen M, Rahman P, Raychaudhuri S, Gottlieb A, Chou R, Honczarenko M, Soule B, Mehta S, Nishandar T, Tripathi A, Kothekar M. Long-Term Safety of Tildrakizumab Through Week 208 in Patients With Psoriatic Arthritis: Results From the Phase 2b Open-Label Extension Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-of-tildrakizumab-through-week-208-in-patients-with-psoriatic-arthritis-results-from-the-phase-2b-open-label-extension-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-of-tildrakizumab-through-week-208-in-patients-with-psoriatic-arthritis-results-from-the-phase-2b-open-label-extension-study/

.jpg)
.jpg)