Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Risankizumab, an interleukin-23 inhibitor, was efficacious and well tolerated in plaque psoriasis (PsO) and psoriatic arthritis (PsA) clinical trials.The objective of this integrated data analysis of multiple PsO and PsA clinical trials was to report long-term safety of risankizumab in patients with psoriatic disease.
Methods: Integrated risankizumab safety data sets (data cutoff March 25, 2023) were compiled from 20 phase 1–4 clinical trials in PsO and 4 phase 2–3 trials in PsA. Treatment-emergent adverse events (AEs) and AEs of safety interest were reported for patients receiving ≥1 dose of risankizumab. Exposure adjusted event rates are presented as events per 100 patient years (E/100 PY).
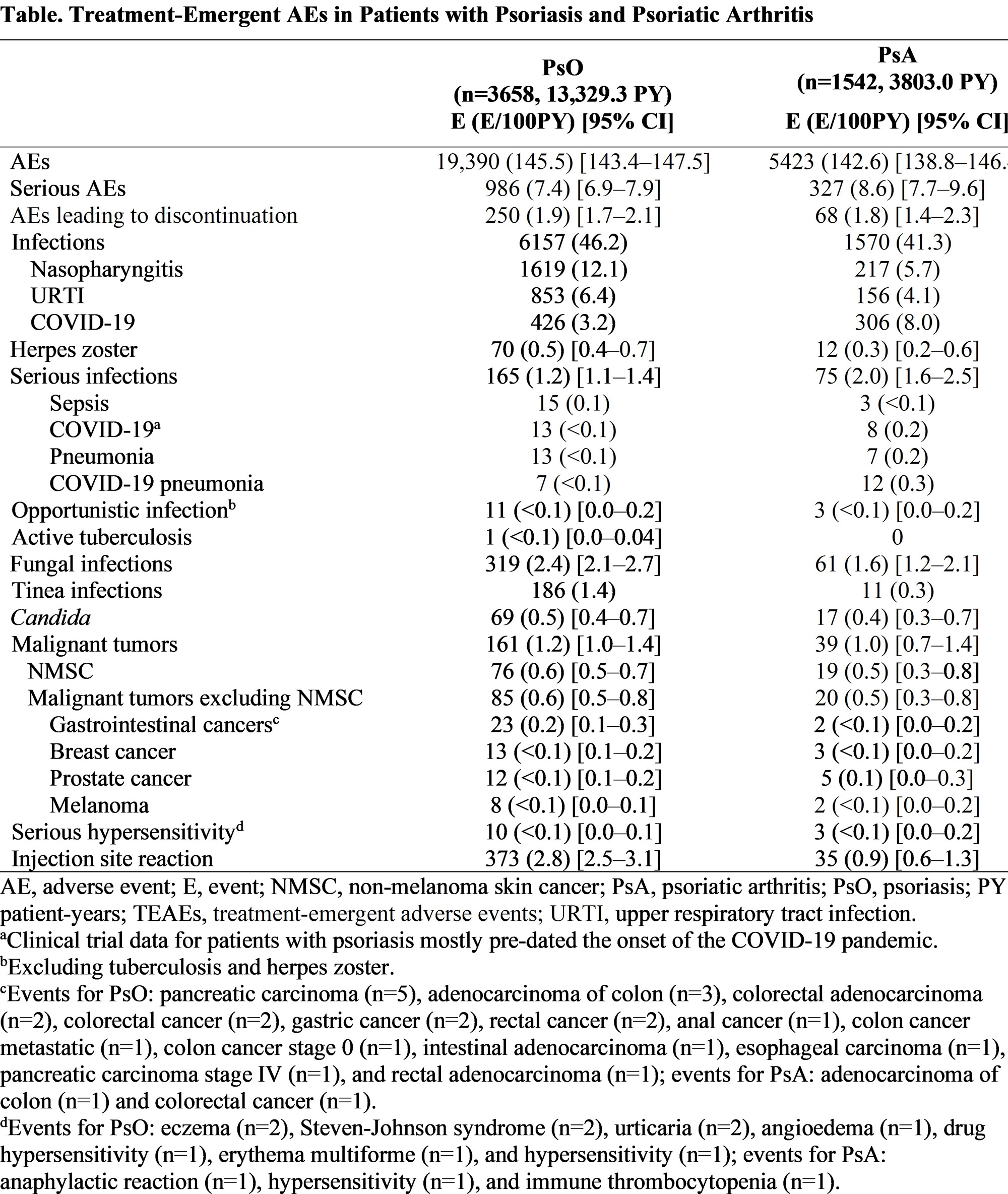
Results: Among 3658 patients with PsO (13,329.3 PY exposure), median (range) of treatment duration was 4.1 years (81 days–8.8 years); among 1542 patients with PsA (3803.0 PY exposure), median (range) treatment duration was 2.8 years (84 days–4.0 years). Rates of treatment-emergent AEs (145.5 events [E]/100PY), serious AEs (7.4 E/100PY), and AEs leading to discontinuation (1.9 E/100PY) in patients with PsO were similar to those in patients with PsA (142.6 E/100PY, 8.6 E/100PY, and 1.8 E/100PY, respectively; Table). Similar rates of infections (46.2 and 41.3 E/100 PY) and serious infections (1.2 and 2.0 E/100PY) were reported among PsO and PsA groups, respectively. Nasopharyngitis (12.1 E/100PY), upper respiratory infection (6.4 E/100PY) and COVID-19 (3.2 E/100PY) were the most common infections in PsO and COVID-19 (8.0 E/100PY), nasopharyngitis (5.7 E/100PY) and upper respiratory infection (4.1 E/100PY) were the most common infections in PsA. The most common serious infections were sepsis, COVID-19, and pneumonia in PsO (0.1, < 0.1, and < 0.1 E/100PY, respectively) and COVID-19 pneumonia, COVID-19, and pneumonia in PsA (0.3, 0.2 and 0.2 E/100PY, respectively). Rates of opportunistic infections excluding tuberculosis (both < 0.1 E/100PY) and herpes zoster (0.5 and 0.3 E/100 PY, respectively) were comparable in PsO and PsA. Rates of non-melanoma skin cancer (NMSC) were 0.6 and 0.5 E/100PY and malignant tumors excluding NMSC were 0.6 and 0.5 E/100PY in PsO and PsA, respectively.
Conclusion: Rates of AEs, AEs of safety interest, and AEs leading to discontinuation remained low in this largest and longest safety reporting for risankizumab in patients with psoriatic disease to date. Rates of AEs of safety interest were within reported benchmarks for both PsO and PsA. Rates of COVID-19 infection were as expected. Overall, these results support the safety profile of risankizumab for the long-term treatment of patients with psoriatic disease.
To cite this abstract in AMA style:
Gordon K, Blauvelt A, Bachelez H, Coates L, Kaplan B, Koetse W, Drogaris L, Sinvhal R, Papp K. Long-Term Safety of Risankizumab in Patients with Psoriatic Disease: Integrated Analysis of Psoriasis and Psoriatic Arthritis Clinical Trial Data [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-of-risankizumab-in-patients-with-psoriatic-disease-integrated-analysis-of-psoriasis-and-psoriatic-arthritis-clinical-trial-data/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-of-risankizumab-in-patients-with-psoriatic-disease-integrated-analysis-of-psoriasis-and-psoriatic-arthritis-clinical-trial-data/