Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The 52-week Phase III MIRRA trial demonstrated the safety and efficacy of anti-IL-5 mepolizumab in patients with EGPA. However, longer-term safety data are limited. This analysis assessed the safety of mepolizumab using pooled data from two Long-term Access Programmes (LAP; NCT03298061/MEA116841, 201607), open-label extensions of MIRRA.
Methods: Patients with EGPA were enrolled in LAP if on an oral corticosteroid dose ≥5mg/day after the end of MIRRA; all patients received mepolizumab 300mg subcutaneous every 4 weeks + standard of care. Follow-up was until discontinuation or after licensing of mepolizumab for EGPA in the patient’s country. Treatment exposure, on-treatment adverse events (AEs) and serious AEs (SAEs) are reported.
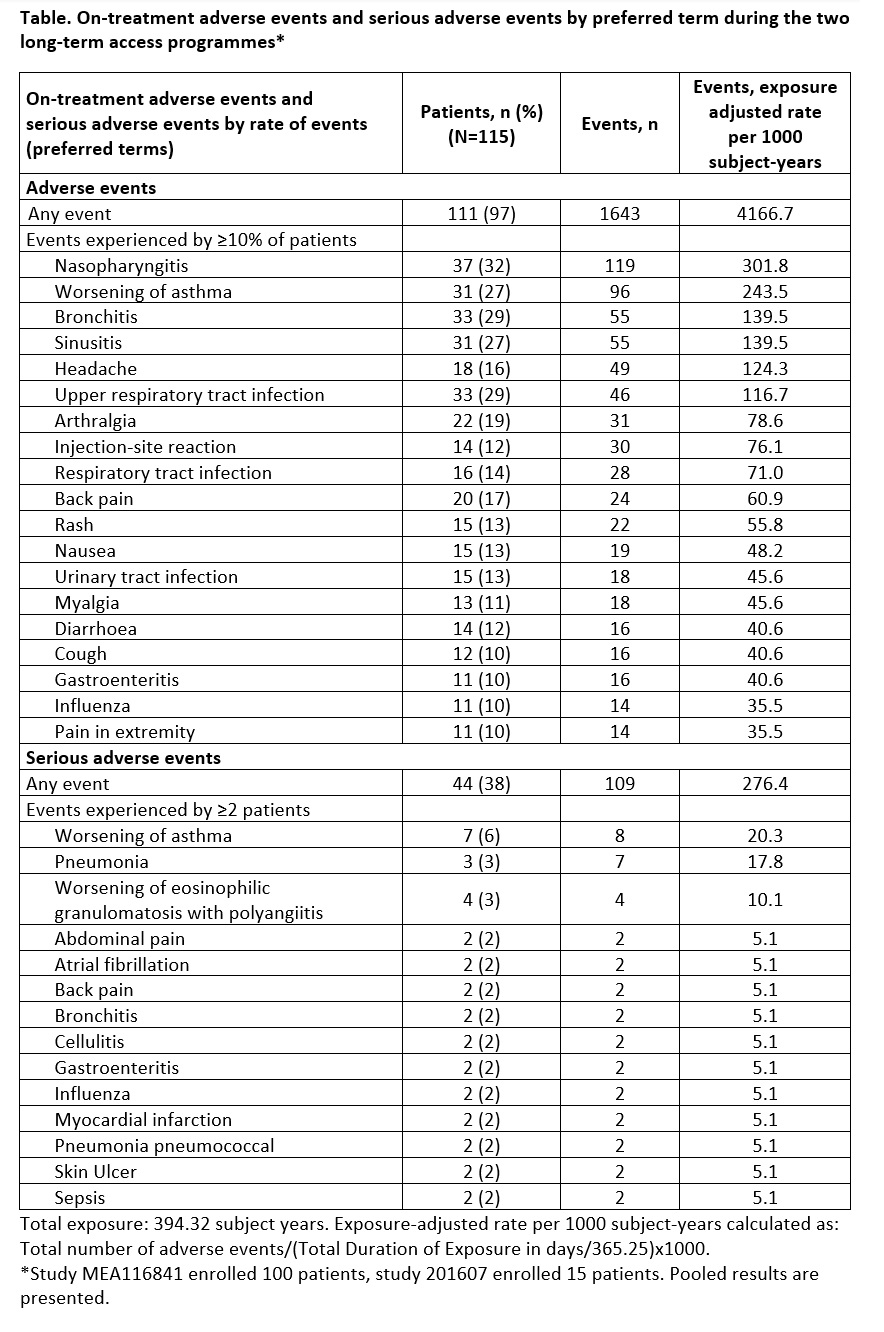
Results: In total 115 patients enrolled in two LAP. Mean (SD) age: 49.7 (13.8) years; 68% completed treatment; 3% discontinued due to an AE. Total exposure was 394.32 subject-years (median [min–max]: 28 [1–91] months). AEs and SAEs occurred in 97% (38% drug-related) and 38% (5% drug-related) of patients; 2 patients had fatal SAEs (non-drug-related). The most common SAE reported was worsening asthma (6%) (Table).
Conclusion: Long-term safety data for mepolizumab (≤7.6 years) are consistent with its known safety profile. The high completion rate in LAP suggests mepolizumab is well tolerated with a positive benefit:risk profile.
Funding: GSK (MEA116841, 201607)
Abstract previously submitted to European Respiratory Society (ERS) 2024 Annual Scientific Meeting.
To cite this abstract in AMA style:
Wolff G, Wechsler M, Silver J, Price R, Verghis R, Weller P, Merkel P, Corbridge T, Khoury P. Long-Term Safety of Mepolizumab in Eosinophilic Granulomatosis with Polyangiitis (EGPA): Pooled Results from Two Open-Label Extension Studies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-of-mepolizumab-in-eosinophilic-granulomatosis-with-polyangiitis-egpa-pooled-results-from-two-open-label-extension-studies/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-of-mepolizumab-in-eosinophilic-granulomatosis-with-polyangiitis-egpa-pooled-results-from-two-open-label-extension-studies/