Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Ixekizumab (IXE) is a high-affinity, monoclonal antibody targeting IL-17A and is approved for the treatment of psoriasis (PsO), psoriatic arthritis (PsA), ankylosing spondylitis (AS), and non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation. We report long-term, end-of-study-program, safety outcomes in adult patients with PsO, PsA and axSpA who received at least one dose of IXE over 5 years (PsO) or 3 years (PsA and axSpA).
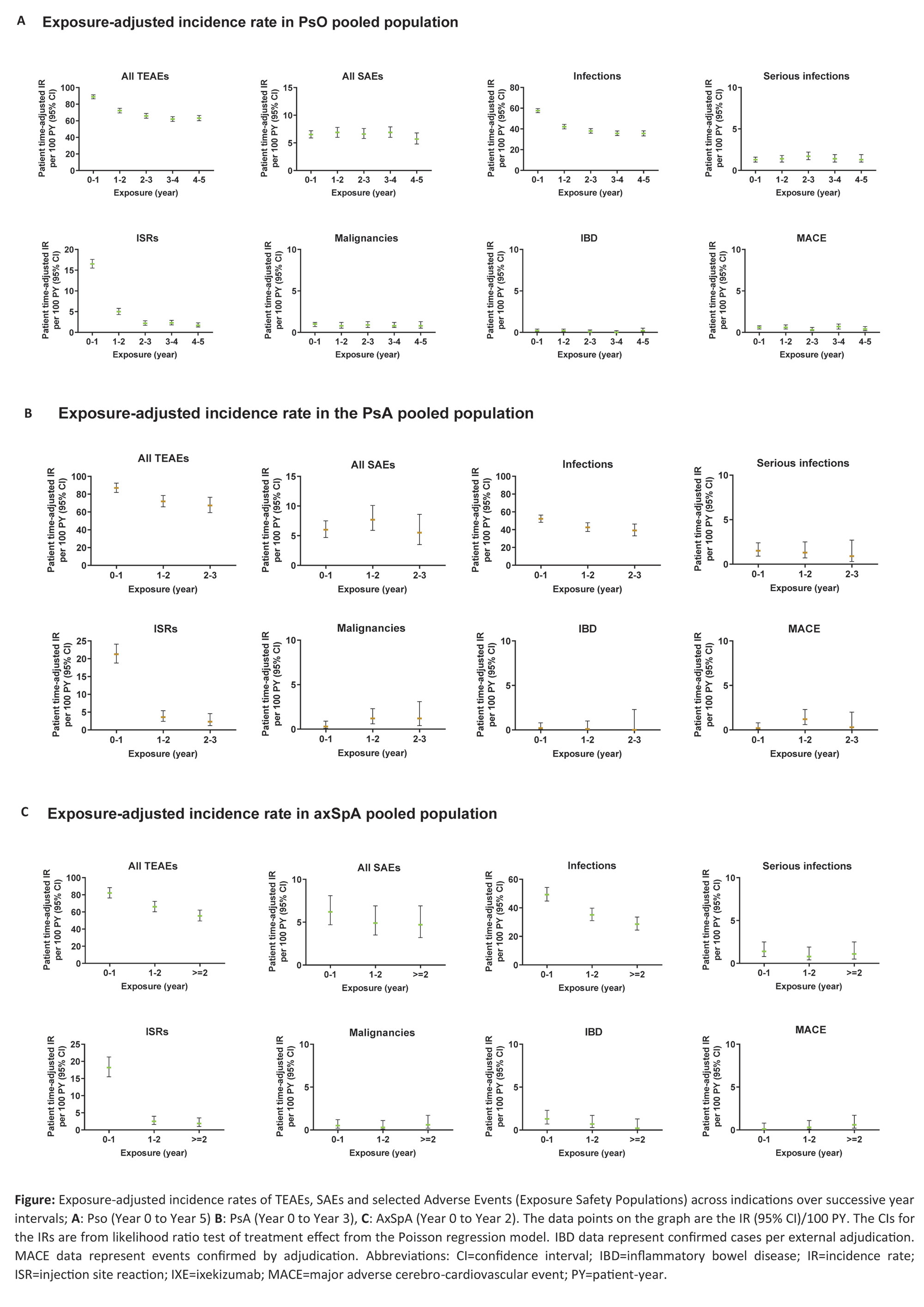
Methods: An integrated safety analysis consisting of data from 25 randomised clinical trials (RCTs; 17 PsO, 4 PsA, 4 axSpA) was used to examine long-term safety of IXE. Rates of treatment-emergent adverse events (TEAEs), serious AEs (SAEs) and AEs of special interest were analyzed for all pooled studies by years of therapy and overall through March 2022, and reported as exposure-adjusted incidence rates (IRs) per 100 patient-years (PY) at successive year intervals. Additional safety outcomes included selected safety topics of interest (among others).
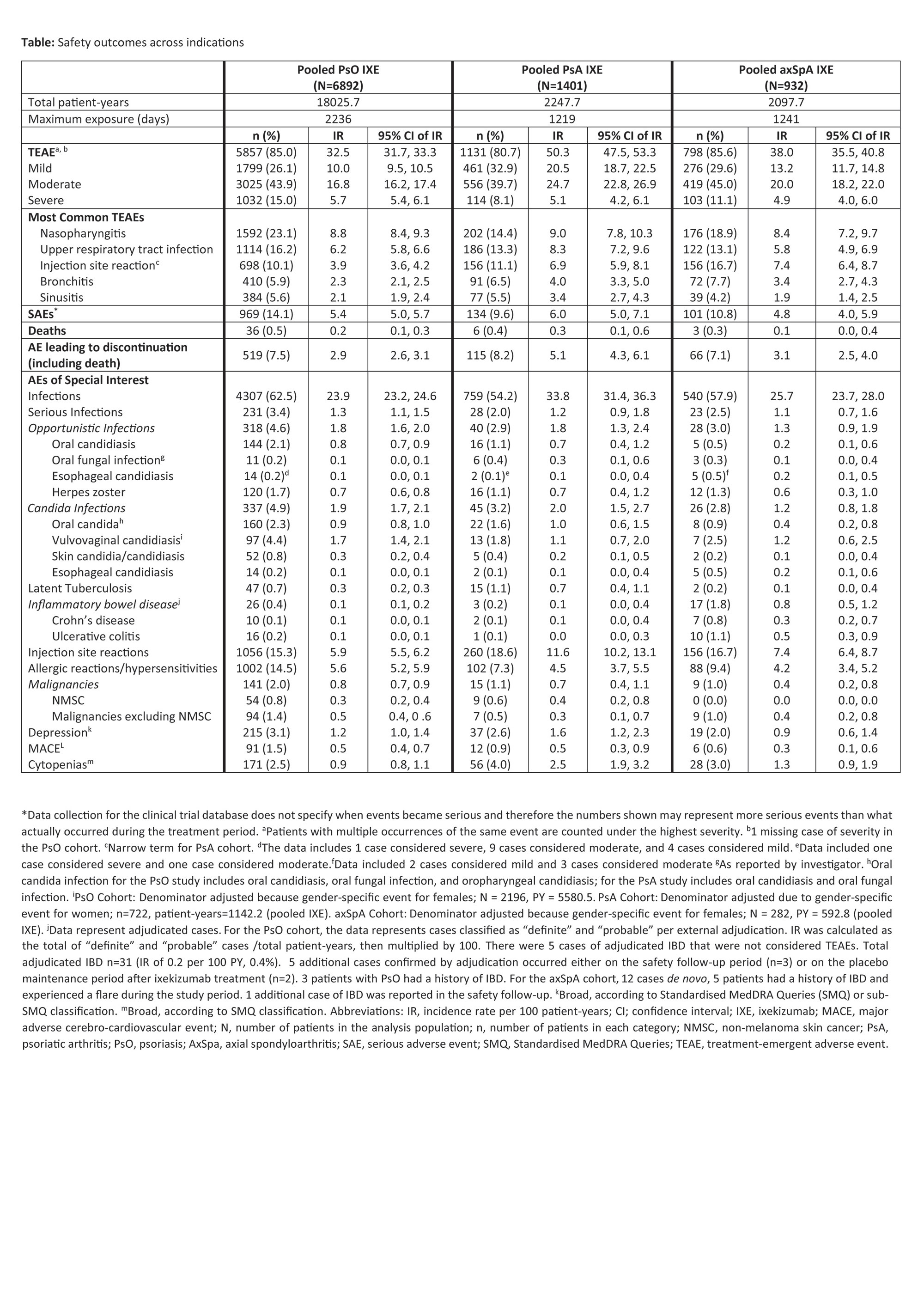
Results: A total of 6892 patients with PsO, 1401 patients with PsA, and 932 patients with axSpA, with a cumulative IXE exposure of 18025.7 PY for PsO, 2247.7 PY for PsA, and 2097.7 PY for axSpA were included in this analysis (Table, Figure). The IRs per 100 PY for any TEAE were as follows; patients with PsO=32.5, PsA=50.3, axSpA=38.0. The most commonly reported TEAEs were nasopharyngitis (PsO, IR=8.8; PsA, IR=9.0; axSpA IR=8.4) and upper respiratory tract infection (PsO, IR=6.2; PsA, IR=8.3; axSpA IR=5.8). Serious AEs were reported by 969 patients with PsO (IR=5.4), 134 patients with PsA (IR=6), and 101 patients with axSpA (IR=4.8). Forty-five deaths were reported; (PsO=36 [IR=0.2]; PsA=6 [IR= 0.3]; axSpA=3 [IR=0.1]). The IRs per 100 PY of discontinuation from the study drug due to AE were as follows: PsO, 2.9; PsA, 5.1; axSpA, 3.1. IRs of injection site reactions were: PsO, 5.9; PsA, 11.6; axSpA, 7.4. IRs of allergic reactions were: PsO, 5.6; PsA, 4.5; axSpA, 4.2. IRs of serious infections were low (PsO, IR=1.3; PsA, IR=1.2; axSpA, IR=1.1). IRs of Candida were low across all indications (PsO, 1.9; PsA, 2.0; axSpA, 1.2), as were IRs of opportunistic infections (PsO, 1.8; PsA, 1.8; axSpA, 1.3). IRs were also low across all indications for depression, major adverse cerebro-cardiovascular events and malignancies (all IRs ≤1.6, Table, Figure). Cases of inflammatory bowel disease (IBD) were uncommon (IRs ≤0.8 across indications (Table, Figure)).
Conclusion: In this updated analysis with 18025.7 PY for PsO, 2247.7 PY for PsA, and 2097.7 PY for axSpA, IXE maintained a long-term safety profile up to 5 years, consistent with previous reports1-7.
REFERENCES: 1. Mease P et al. Arthritis Care Res (Hoboken) 2019;71(3):367-78. 2. Combe B et al. Arthritis Res Ther 2020;22(1):14. 3. Genovese MC et al. Rheumatology (Oxford) 2020;59(12):3834-44. 4. Armstrong A, et al. Dermatol Ther (Heidelb). 2020;10(1):133-150. 5. Strober B, et al. J Am Acad Dermatol. 2017;76(3):432-40 e17. 6. Langley RG et al. J Eur Acad Dermatol Venereol. 2019;33(2):333-9. 7. Griffiths et al. Dermatol Ther (Heidelb). 10.1007/s13555-022-00743-9.
To cite this abstract in AMA style:
Deodhar A, Blauvelt A, Schwartzman S, Salvarani C, Feely M, Kronbergs A, Eberhart N, Zhu D, Mevel E, Holzkämper T, Krishnan E, Lebwohl M, Rahman P, Marzo-Ortega H. Long-term Safety of Ixekizumab in Adult Patients with Psoriasis, Psoriatic Arthritis, and Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-of-ixekizumab-in-adult-patients-with-psoriasis-psoriatic-arthritis-and-axial-spondyloarthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-of-ixekizumab-in-adult-patients-with-psoriasis-psoriatic-arthritis-and-axial-spondyloarthritis/