Session Information
Date: Tuesday, November 9, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster III: Psoriatic Arthritis II (1801–1835)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Guselkumab (GUS), a targeted IL-23 inhibitor, demonstrated significant improvements in signs and symptoms of psoriatic arthritis (PsA) and a favorable safety profile through week (W) 24 in one Phase (Ph)21 and two Ph3 (DISCOVER [D]-1&2)2-4 randomized controlled trials (RCTs). In this study, we assessed GUS safety by pooling data across the 1-year (Y) Ph2, 1-Y D-1, and 2-Y D-2 RCTs.
Methods: Patients (Pts) with active PsA (≥3% BSA affected by psoriasis in Ph2; ≥3 tender/swollen joints and CRP ≥0.3 mg/dL in Ph2 and D-1; ≥5 tender/swollen joints and CRP ≥0.6 mg/dL in D-2), biologic naïve except 13/149 Ph2 and 118/381 D-1 pts who received prior 1-2 TNF inhibitors (TNFi), were randomized to subcutaneous GUS 100 mg at W0, W4, and every 8 weeks (Q8W) or placebo (PBO) in Ph2 or to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, Q8W; or PBO in D-1 & D-2. At W24, PBO pts switched to GUS 100 mg Q8W (Ph2) or Q4W (D-1 & D-2). In these pooled post-hoc analyses, adverse events (AEs; number standardized for 100 patient-years of follow-up [PY]), laboratory investigations (abnormalities classified by National Cancer Institute Common Terminology Criteria for AEs [NCI-CTCAE] grade), and injection site reactions (ISRs) were reported through W56 for the Ph2 trial, W60 for D-1, and W112 for D-2.
Results: Across the 3 RCTs, 1,229 pts with active PsA received GUS 100 mg (725 Q4W, 504 Q8W) and were followed for an average of 1.5 Y, representing 1871 PY. Incidences of AEs, serious AEs, infections, serious infections, discontinuations due to an AE, malignancies, and major adverse cardiovascular events were similar between PBO and GUS through W24. No increased rates were seen with up to 2 Y of GUS, except for a somewhat higher rate of SAEs and serious infections in the GUS 100 mg Q8W group during long-term follow-up, although confidence intervals overlapped with those seen during the PBO-controlled period (Table 1). The majority of GUS-treated pts with elevated aminotransferases and blood bilirubin had NCI-CTCAE Grade 1/2, with very few Grade 3 and no Grade 4, elevations. The proportions of pts with elevated aminotransferases at W24 were somewhat higher in the GUS Q4W vs Q8W/PBO groups; no unexpected increase was observed with longer duration of treatment. Elevations were more common in pts with vs without methotrexate (MTX) use at baseline. The proportions of pts with Grade 1 elevated bilirubin were higher in GUS vs PBO groups; few Grade 2 and no Grade 3/4 elevations were seen (Table 2). ISRs occurred in similar proportions of GUS (1%) and PBO (0.5%) pts at W24, with no disproportional increase with up to 2 Y of GUS.
Conclusion: In pts with active PsA pooled across 3 RCTs, GUS demonstrated a favorable safety profile through up to 2 Y of treatment; the GUS safety profile in PsA was comparable to that observed through up to 5 Y of GUS in pts with moderate-to-severe psoriasis.5
References
1. Deodhar A et al. Lancet. 2018;391(10136):2213
2. Ritchlin C et al. RMD Open. 2021;7(1):e001457
3. McInnes I et al. Arthritis Rheumatol. 2021;73(4):604
4. Rahman P et al. J Rheum. doi:10.3899/jrheum.201532
5. Griffiths CEM et al. Coastal Dermatol Symposium 2020; Oct 15–16
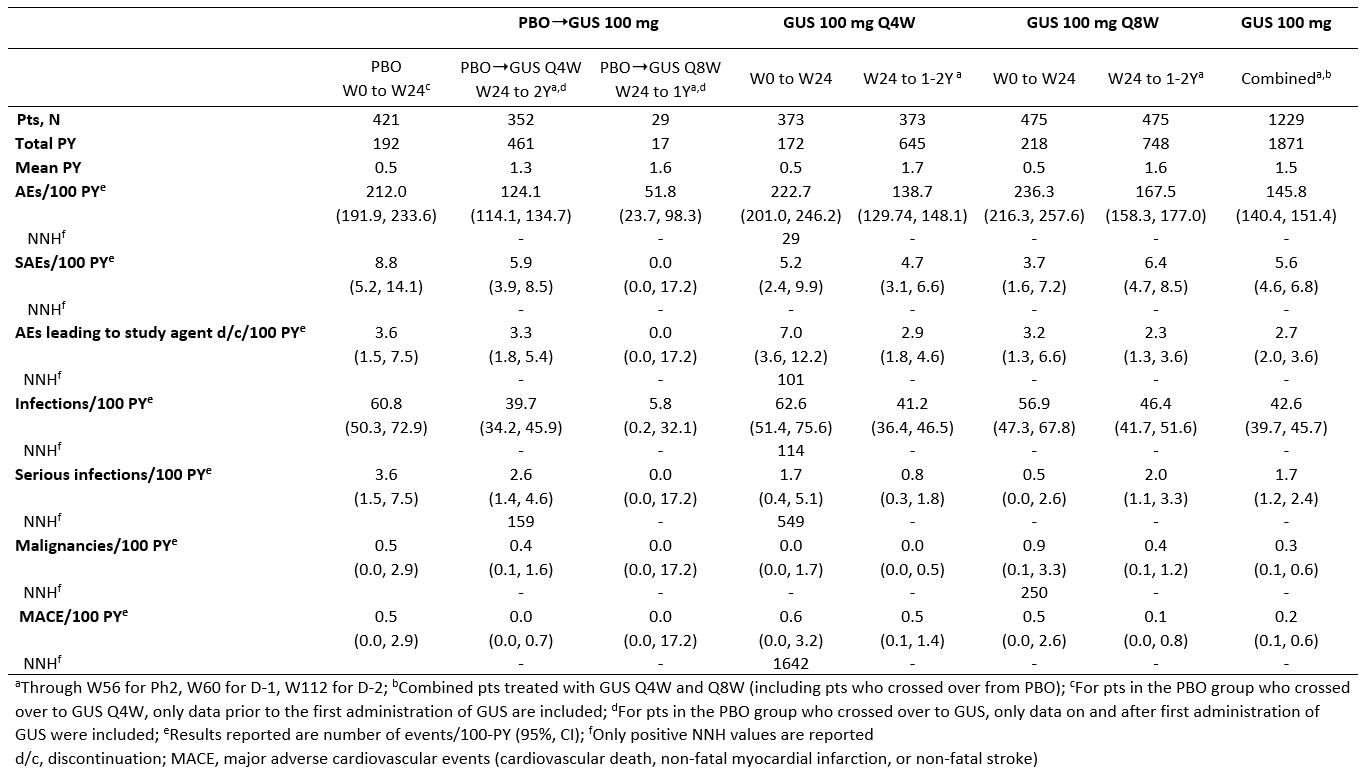 Table 1. Number of adverse events per 100 PY (95% CI)
Table 1. Number of adverse events per 100 PY (95% CI)
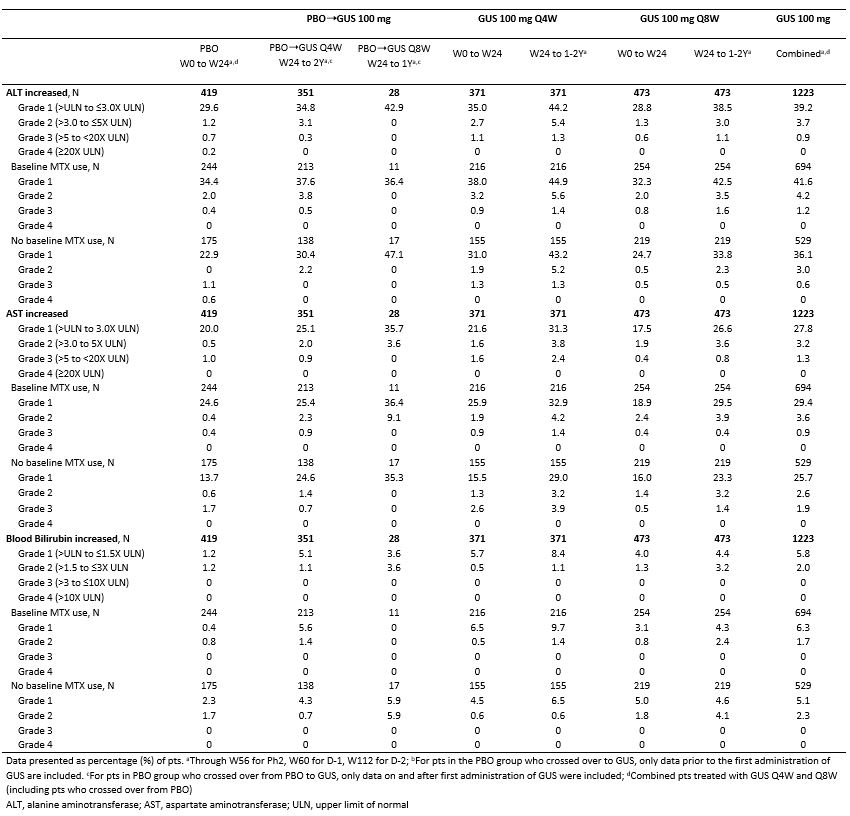 Table 2. Proportions of pts (%) with clinical laboratory abnormalities according to maximum NCI-CTCAE toxicity grade and by baseline MTX use
Table 2. Proportions of pts (%) with clinical laboratory abnormalities according to maximum NCI-CTCAE toxicity grade and by baseline MTX use
To cite this abstract in AMA style:
Rahman P, Ritchlin C, Mease P, Helliwell P, Boehncke W, McInnes I, Shawi M, Marrache A, Kollmeier A, Xu X, Yu J, Wang Y, Sheng S, Gottlieb A. Long-term Safety of Guselkumab (TREMFYA®) in Patients with Active Psoriatic Arthritis: Pooled Results from 3 Randomized Clinical Trials Through up to 2 Years [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-of-guselkumab-tremfya-in-patients-with-active-psoriatic-arthritis-pooled-results-from-3-randomized-clinical-trials-through-up-to-2-years/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-of-guselkumab-tremfya-in-patients-with-active-psoriatic-arthritis-pooled-results-from-3-randomized-clinical-trials-through-up-to-2-years/
