Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Psoriasis and PsA are associated with multiple comorbidities such as cardiovascular disease and metabolic syndrome. These comorbidities may render patients prone to developing adverse events (AEs) during treatment. In contrast to randomized controlled trials (RCT), patients with comorbidities frequently receive biologic treatment in real life, and long-term safety profiles may differ from RCT findings. Here, we analyze the long-term safety of biologic treatments from the real-world PsABio study, including the influence of baseline comorbidities on treatment effects.
Methods: PsABio (NCT02627768) was a multinational, observational study in patients with PsA prescribed either ustekinumab (UST, an IL-12/23 inhibitor) or a TNF inhibitor (TNFi) as 1st, 2nd or 3rd line treatment. The safety set included all patients with baseline and any available follow-up data. The frequency of new AEs or aggravation of existing comorbidities was analyzed during 6-monthly follow-up periods until study end at a maximum of 3 years (36 ± 3 months) of follow-up. AEs were summarized for all treatments that started prior to the AE and included events that occurred within a 91-day period (risk window) after treatment stop. All neoplasm events, excluding the ones occurring within 12 months of treatment start, are included irrespective of when they occurred after treatment stop.
Results: Safety data exist for 494 patients treated with UST and 557 treated with TNFi during the 36-month study period (this includes patients who switched from one treatment to the other). Baseline characteristics, treatment data and comorbidities are presented in Table 1. The UST group were older, had more comorbidities (especially cardiometabolic disease) and later line of bDMARD treatment vs the TNFi group, who had higher MTX use. UST-treated patients were followed for a total of 991.3 patient-years (PY), and TNFi-treated patients for 1232.9 PY; the mean follow-up time per patient for the UST group was 2.0 (±1.0) years vs 2.2 (±0.9) years for the TNFi group. At least one (non-neoplasm) AE was recorded in 34.6% of UST and 39.7% of TNFi-treated patients, with 6.3% and 7.2%, respectively, recording at least 1 serious AE (SAE). Malignancies were recorded in 3 (0.6%) UST (colon cancer, malignant neoplasm of eye, and prostate cancer) and 4 (0.7%) TNFi (bladder neoplasm, colon cancer, malignant urinary tract neoplasm, and squamous cell carcinoma of skin) patients. In the subgroup analysis by baseline characteristics and comorbidities, UST-treated patients generally tended to have a lower risk of clinically relevant AEs vs TNFi-treated patients in several subgroups (Figure 1a). The risk of infections was also lower with UST vs TNFi for the overall group and for several subgroups (Figure 1c). No clinically relevant differences were detected for SAEs (Figure 1b).
Conclusion: The PsABio study demonstrated an acceptable long-term safety profile of treatment with UST and TNFi in patients with PsA in a real-world setting.
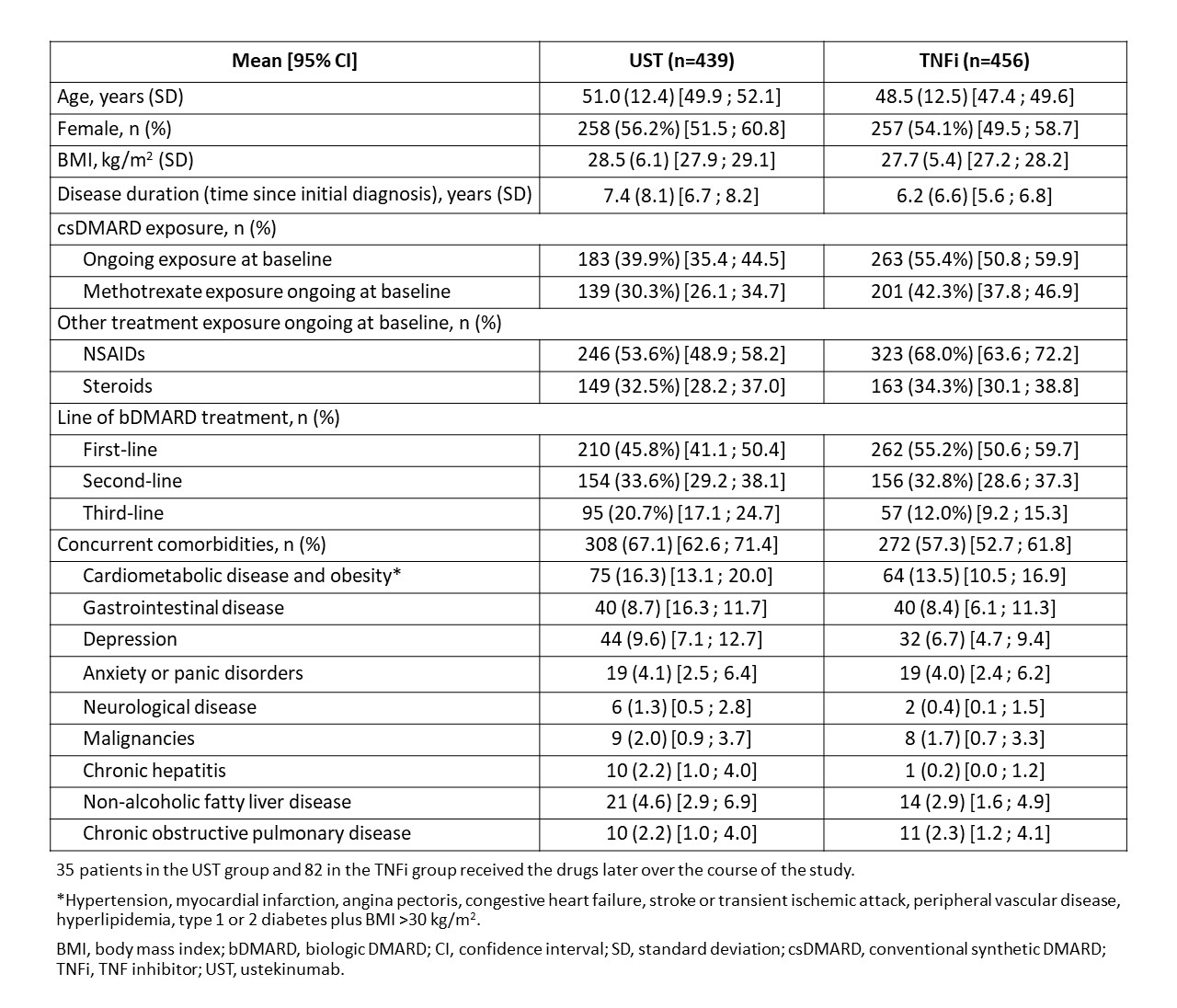 Table 1: Baseline demographics, treatment characteristics and comorbidities of patients with PsA treated with ustekinumab and TNFi
Table 1: Baseline demographics, treatment characteristics and comorbidities of patients with PsA treated with ustekinumab and TNFi
 Figure 1: Exposure-adjusted incidence rate per 100 PY at risk (95% CI) for the occurrence of: (a) adverse events; (b) serious adverse events; and (c) infections in patients receiving UST and TNFi in the PsABio study. AE, adverse event; BMI, body mass index; BSA, body surface area; CI, confidence interval; CV, cardiovascular; IR, incidence rate; PY, patient-year; SAE, serious adverse event; TNFi, TNF inhibitor; UST, ustekinumab.
Figure 1: Exposure-adjusted incidence rate per 100 PY at risk (95% CI) for the occurrence of: (a) adverse events; (b) serious adverse events; and (c) infections in patients receiving UST and TNFi in the PsABio study. AE, adverse event; BMI, body mass index; BSA, body surface area; CI, confidence interval; CV, cardiovascular; IR, incidence rate; PY, patient-year; SAE, serious adverse event; TNFi, TNF inhibitor; UST, ustekinumab.
To cite this abstract in AMA style:
Siebert S, Bergmans P, de Vlam K, Gremese E, Joven-Ibáñez B, Korotaeva T, Noël W, Nurmohamed M, Sfikakis P, Theander E, Gossec L, Smolen J. Long-Term Safety Data for IL-12/23 Inhibitor (Ustekinumab) or Tumor Necrosis Factor Inhibitor in Patients with Psoriatic Arthritis from a Real-World Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-data-for-il-12-23-inhibitor-ustekinumab-or-tumor-necrosis-factor-inhibitor-in-patients-with-psoriatic-arthritis-from-a-real-world-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-data-for-il-12-23-inhibitor-ustekinumab-or-tumor-necrosis-factor-inhibitor-in-patients-with-psoriatic-arthritis-from-a-real-world-study/
