Session Information
Date: Sunday, November 12, 2023
Title: (0510–0542) Spondyloarthritis Including Psoriatic Arthritis – Treatment: AxSpA Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Bimekizumab (BKZ) is a monoclonal IgG1 antibody that selectively inhibits IL‑17F in addition to IL‑17A. A previous analysis of pooled safety data from the placebo-controlled period of four phase 3 trials was conducted in patients (pts) with axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) to Week (Wk) 16.1 Here, we present long-term pooled safety data for BKZ in axSpA and PsA pt populations, across phase 2b/3 trials and all study periods.
Methods: We report two pooled analyses, each comprising three phase 2b/3 trials, and their open-label extensions, in pts with active axSpA (non-radiographic [nr]-axSpA and radiographic [r]-axSpA, i.e., ankylosing spondylitis)2 and active PsA, respectively (Figure).1,3–5
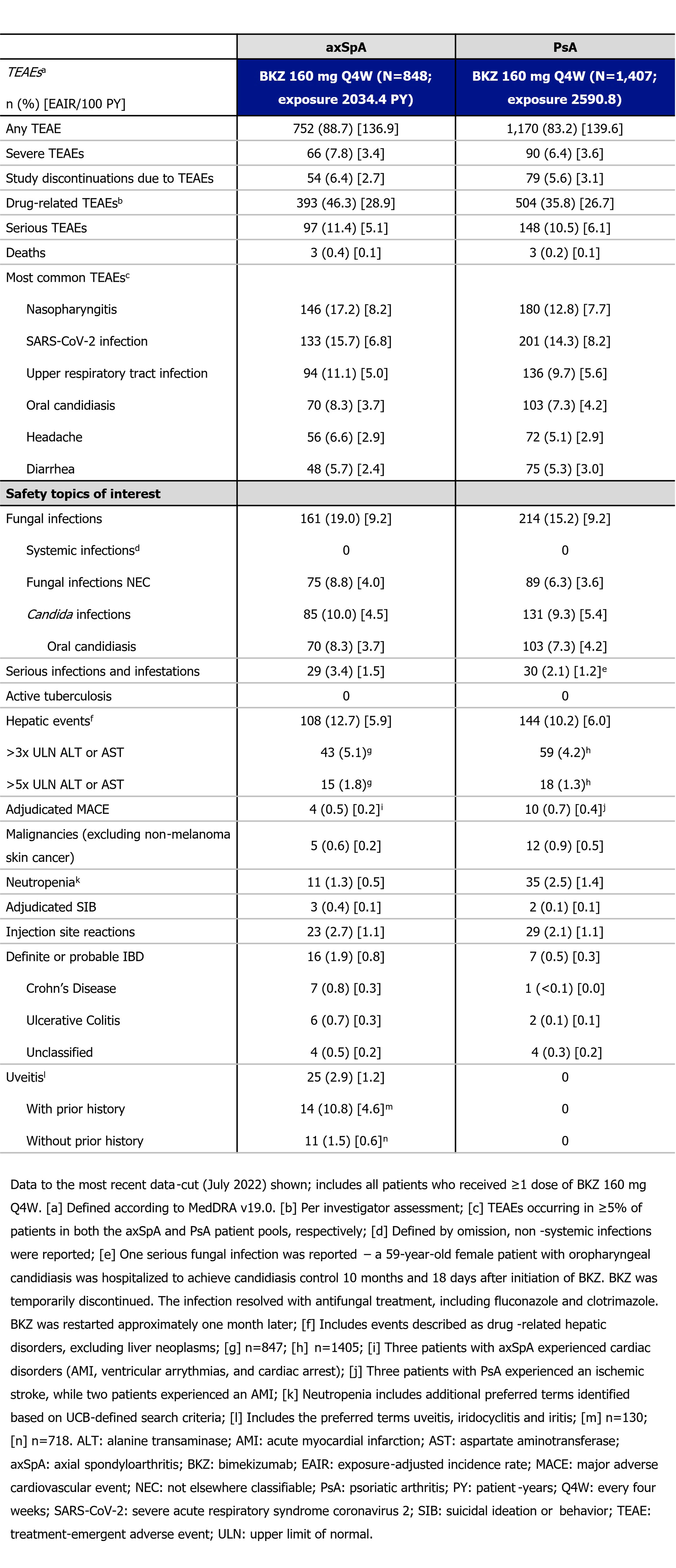
The number, proportion, and exposure-adjusted incidence rates (EAIRs) per 100 pt-years (PY) of treatment-emergent adverse events (TEAEs) are reported for all pts who received ≥1 dose of BKZ 160 mg every four weeks (Q4W). Data are reported to the most recent data-cut (July 2022) across all treatment periods.
Results: The axSpA and PsA safety pools included 848 pts (2034.4 PY) and 1,407 pts (2590.8 PY), respectively. The most frequently reported TEAEs in pts with axSpA and PsA were nasopharyngitis, SARS-CoV-2 infection, and upper respiratory tract infection (Table). See Table for safety topics of interest.
Fungal infections were reported in 375 pts (axSpA: 161 [19.0%]; PsA: 214 [15.2%]), leading to discontinuation in 19 pts (7 axSpA [0.8%], 12 PsA [0.9%]); no fungal infections were systemic in nature. Most fungal infections were oral, and the vast majority of oral fungal infections were mild to moderate in severity (axSpA: 162 [99.4%]; PsA: 231 [99.6%]). Two pts experienced severe oral fungal infections (axSpA: 1 [0.1%]; PsA: 1 [0.1%]), which resolved in both cases with treatment. Candida fungal infections were seen in a similar proportion of pts with axSpA and PsA (Table).
Serious infections and infestations occurred in 59 pts (axSpA: 29 [3.4%]; PsA: 30 [2.1%], Table). One serious fungal infection (oropharyngeal candidiasis) was reported in a pt with PsA (1 [< 0.1%]), which resolved with treatment (further details provided in Table).
Adjudicated IBD (definite or probable) occurred in 16 pts with axSpA (EAIR/100 PY: 0.8) and 7 pts with PsA (EAIR/100 PY: 0.3). Uveitis occurred in 25 pts with axSpA (EAIR/100 PY: 1.2), while no uveitis cases were reported in pts with PsA (Table).
Fourteen pts experienced an adjudicated major adverse cardiovascular event (axSpA: 4 [EAIR/100 PY: 0.2]; PsA: 10 [EAIR/100 PY: 0.4]). Five pts experienced events adjudicated as suicidal ideation/behavior (axSpA: 3 [EAIR/100 PY: 0.1]; PsA: 2 [EAIR/100 PY: 0.1]), while no cases of active tuberculosis were reported (Table).
Conclusion: The long-term safety profile of BKZ in pts with axSpA and PsA is consistent with prior studies.1 As expected, due to inhibition of IL-17, oral candidiasis featured among the safety signals reported.
References: 1. Poddubnyy D. Ann Rheum Dis. 2023;82(suppl 1); 2.Boel A. Ann Rheum Dis. 2019;78:1545–9; 3. Baraliakos X. Arthritis Rheumatol. 2022;74(suppl 9); 4. Ritchlin C. Arthritis Rheumatol. 2022;74(suppl 9); 5. Merola J. Ann Rheum Dis. 2022;81:167–9.
To cite this abstract in AMA style:
Mease P, Poddubnyy D, Orbai A, Warren R, Fleurinck C, Bajracharya R, Ink B, Massow U, Shende V, Shepherd-Smith J, Peterson L, White K, Landewé R, Gensler L. Long-term Safety and Tolerability of Bimekizumab in Patients with Axial Spondyloarthritis and Psoriatic Arthritis: Results from Pooled Phase 2b/3 Studies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-and-tolerability-of-bimekizumab-in-patients-with-axial-spondyloarthritis-and-psoriatic-arthritis-results-from-pooled-phase-2b-3-studies/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-tolerability-of-bimekizumab-in-patients-with-axial-spondyloarthritis-and-psoriatic-arthritis-results-from-pooled-phase-2b-3-studies/