Session Information
Date: Tuesday, November 10, 2015
Title: Rheumatoid Arthritis-Small Molecules, Biologics and Gene Therapy V: Immunogenecity
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
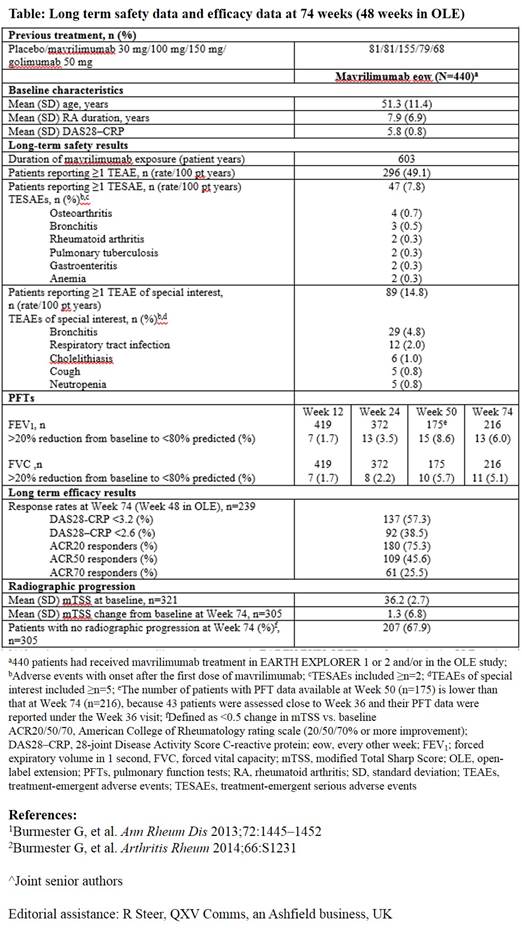
Background/Purpose: Modulating macrophage function through GM–CSF is a novel therapeutic approach for RA. Mavrilimumab, a fully human monoclonal antibody, which targets GM–CSFR-α, has demonstrated efficacy and an acceptable safety profile in prior 12- and 24‑week studies.1,2This analysis evaluated the long‑term (LT) safety and efficacy of mavrilimumab through 74 weeks of treatment.
Methods: This open-label extension (OLE) study (NCT01712399) enrolled adult RA patients (pts) who had completed the EARTH EXPLORER 12 and 2 (NCT01715896) Phase IIb studies or were rescued as inadequate responders at a predefined time point. Pts received subcutaneous mavrilimumab 100 mg every other week (eow), consistent with the highest dosage in the Phase IIa study.1The primary objective was to assess the LT risk:benefit ratio of mavrilimumab via evaluation of i) Treatment emergent adverse events (TEAEs), TE serious AEs (TESAEs), and pulmonary function tests (PFTs), and ii) Exploratory LT efficacy endpoints (DAS28–CRP/ACR responses). AEs with onset date after mavrilimumab had been started are presented. Change from baseline (BL) in modified Total Sharp Score (mTSS) was used to explore radiographic progression in pts previously included in EARTH EXPLORER 1.
Results: At the 74 week (includes original study) cutoff, 391 pts had been enrolled in the OLE study. Of these, 329 (84.1%) continued on treatment and 62 (15.9%) discontinued (due to withdrawal of consent, 9/391 [2.3%]; death [cardiopulmonary failure], 1/391 [0.3%]; adverse event, 7/391 [1.8%]; non study-related site closure, 20/391 [5.1%]; other, 24/391 [6.1%]; lost-to-follow-up, 1/391 [0.3%]). Between the Phase IIb and OLE studies, 440 pts received mavrilimumab, with a safety exposure of 603 pt years (yr) and median (min–max) duration of 1.6 (0.1–2.4) yr. Most common TEAEs (n [/100 pt yr]) were nasopharyngitis (51 [8.45]), bronchitis (36 [5.97]) and hypertension (32 [5.30]); the serious infection rate was 1.82/100 pt yr. PFT demonstrated a generally transient 20% reduction to < 80% of predicted in a few pts (Table). Mavrilimumab demonstrated sustained efficacy with DAS28–CRP <3.2 and <2.6 rates of 57.3% and 38.5%, respectively (Table). After 74 weeks of treatment, 68% of pts showed no radiographic progression (<0.5 change in mTSS vs. BL) (Table).
Conclusion: Mavrilimumab continues to demonstrate a sustained efficacy and safety profile in pts with moderate to severe RA, over the 74 week treatment duration reported. No significant pulmonary signals were observed. Although mavrilimumab 100 mg eow is suboptimal compared with 150 mg eow in DMARD-IR pts,2 efficacy results are comparable with those of previous studies,1,2further validating the use of mavrilimumab to target the GM–CSFR-α.
To cite this abstract in AMA style:
Burmester G, McInnes I, Kremer J, Miranda P, Vencovský J, Godwood A, Albulescu M, Close D, Weinblatt M. Long-term Safety and Efficacy of Mavrilimumab, a Fully Human Granulocyte-Macrophage Colony-Stimulating Factor Receptor-α (GM–CSFR-α) Monoclonal Antibody, in Patients with Rheumatoid Arthritis (RA) [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-mavrilimumab-a-fully-human-granulocyte-macrophage-colony-stimulating-factor-receptor-gmcsfr-monoclonal-antibody-in-patients-with-rheumatoid/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-mavrilimumab-a-fully-human-granulocyte-macrophage-colony-stimulating-factor-receptor-gmcsfr-monoclonal-antibody-in-patients-with-rheumatoid/